Extracorporeal membrane oxygenation in the pre and post lung transplant period
Introduction
Extracorporeal Life Support (ECLS) also known as Extracorporeal Membrane Oxygenation (ECMO) is a life support technology device that performs gas-exchange external to the body by providing cardio-respiratory support for patients with severe respiratory and/or cardiac failure (1). Current evidence supporting use of ECMO in adult patients remains limited. Early randomized trials examining ECMO in the 1970’s failed to show a survival advantage for ECMO with high rates of complications in the ECMO arm (2,3). Subsequent single-center experiences showed no survival benefit (4). However, a recent multicenter, randomized trial investigating the use ECMO for acute respiratory distress syndrome (ARDS) (CESAR) found that referral to centers with ECMO expertise was associated with significant improvement in survival in ARDS compared to previous studies (5). Several retrospective case series and studies since the CESAR trial have reported survival benefits of ECMO in patients with severe ARDS secondary to influenza (6-14).
Despite limited evidence, there has been an increased use of ECMO to support patients with cardio-respiratory failure (15). Proponents of this advanced life support technology believe that its increased utilization and better outcomes are due to improvements in ECMO technology, personnel training, ambulatory practices on ECMO and lung protective ventilation strategies. Another important but controversial aspect of ECMO today is its use as a means to bridge patients to lung transplantation. This evolving practice involves a select few transplant centers who also have experience with ECLS technology. Patients requiring ECMO are more severely ill and thus have a higher lung allocation score (LAS), which is advantageous for lung transplant listing (16). Initial studies after the inception of LAS scoring noted that one-year outcomes in patients with higher LAS (17) and patients bridged with ECMO were lower (18,19). However, more recent studies analyzing the UNOS database have showed that outcomes in ECMO patient’s post-lung transplant may be similar to non-ECMO patients, especially in centers with higher transplant volumes and experience (20,21).
The focus of this article is to provide a concise review of the best practices for utilization of ECMO in the pre- and post-lung transplantation period.
ECMO in the pre-transplant period
Current trends in utilization and outcomes
Regional differences in organ availability, institutional preferences related to the use and management of ECMO, surgeon-specific preferences regarding organ selection and operative technique may have profound effects on the outcomes of ECMO as a bridge to lung transplantation.
According to cumulative global data from the Extracorporeal Life Support Organization (ELSO) from 2016, approximately 10,601 adult patients have been treated with ECLS for respiratory failure since 1990 with a survival to discharge rate of 58% (22). On querying the ELSO database [1990–2016], 1,066 lung transplant recipients and/or patients on the transplant waitlist were supported with ECLS in the pre-, peri or post-operative period with an overall ECLS survival to hospital discharge of 65% (22). The overall utilization of ECLS as a bridge to lung transplant remains miniscule as compared to the total rates of lung transplantation. The United Organ Sharing Network (UNOS), data registry (SRTR data files) reports that, of the total 21,927 patients with lungs transplanted in the United States from 2000–2014, 414 adults have been bridged using ECLS technology (www.UNOS.org). It has been shown that the volume of lung transplantation may determine post-transplant outcomes. Weiss et al. reported a 2% increase in mortality with every percentage decrease in lung transplant center volume in the United States (23). Similar results were seen in patients bridged to transplant using ECLS. Of the 65 centers performing lung transplantation in the United States, only 26 centres utilized ECLS as a bridge to lung transplantation (24). Of these 26 centers, 12 performed one or less transplant using ECLS, while 14 performed >1 lung transplant using ECLS as a bridge (24). Two recent studies examining the UNOS registry showed that the overall one-year survival of patients bridged to lung transplant using ECLS in centers with greater volumes and ECLS experience were significantly higher than those with lower volumes/experience (20,24). Likewise, several single-center studies have reported one-year survivals similar to non-ECMO bridged patients (25-29). These results suggest a trend towards improving short to medium term post-transplant outcomes in ECMO bridged patients, especially at higher volume and more experienced ECLS centers.
LAS score and organ availability
In 2005, UNOS introduced the LAS scoring system, which resulted in improvement in the organ allocation system for lungs, reduced waitlist mortality and time to transplant (16,30). Listed patients with more severe disease requiring higher fraction of inhaled oxygen on respiratory support devices such as mechanical ventilation and/or ECMO (Fio2 on ECMO is usually 100%) receive a higher LAS score (16). Consequently, these patients have a higher priority in receiving organ offers and may have shorter times to transplant. However, several other factors may influence wait times for transplant for patients on ECMO support. It has been noted that local allocation of donor organs to candidates with lower LAS scores over regional candidates with higher LAS may occur (31), thus potentially increasing the wait times on ECMO support. Additionally, surgeon-specific preferences regarding organ selection, complications on ECMO such as bleeding or vascular compromise, poor nutritional status and/or deconditioning leading to temporary waitlist inactivation or human leukocyte antigen (HLA) sensitization due to transfusions may prolong wait times/duration on ECMO. The mean reported duration on ECMO as a bridge to lung transplant is 13–25 days (25). Several case studies have reported that longer ECMO duration may be feasible (32-35). Our center has successfully performed lung transplant on a patient supported with ECMO for 370 days (unpublished data-in review). Thus, it seems that despite the challenges, long-term ECMO support as a bridge to lung transplantation may be feasible.
Candidate selection
Inappropriate patient selection and delayed deployment of ECMO technology have been significant predictors of poor patient outcomes (19,25). Unfortunately, there are no universally accepted guidelines available, and individual institutional practices dominate the decisions to bridge a patient on ECMO. Initial experience in the use of ECMO in pulmonary transplantation was primarily for post-operative primary graft dysfunction or acute rejection episodes (36-38). Generally, it is agreed that ECMO be deployed in the pre-transplant phase in patients who are already listed and suffer an acute decompensation of their existing lung disease.
Additionally, these patients should have a good nutritional status, moderate functional status and/or the perceived ability to rehabilitate while on ECMO support (19,27). Early consideration of ECMO in the management algorithm of these decompensating patients appears to result in more favorable outcomes. Table 1 details some considerations for selection of candidates as a bridge to lung transplantation. Several centers have reported use of ECMO as a bridge to transplantation in acute respiratory failure patients who previously did not have a lung disorder (25,32). In our experience, it is possible to perform expedited transplant workup especially in younger patients supported with ECMO and bridge them to transplantation.

Full table
ECMO cannulation strategies
Several aspects have to be considered to choose the optimal ECMO cannulation strategy for bridging patients to pulmonary transplantation. The objective of any cannulation strategy is not only to provide respiratory and/or cardiac support but also to facilitate early ambulation and minimize undue complications, while reducing common complications that occur with mechanical ventilation. In addition, the average wait times to receive organs on ECMO may also determine the choice of cannulation. Centers with shorter wait times may choose peripheral veno-venous (VV) or veno-arterial (VA) configuration versus a double lumen internal jugular cannula (DLC) or a central VA ECMO approach.
In cases of isolated respiratory failure, a VV configuration using a dual-lumen catheter (DLC) is preferred to facilitate ambulation (25,29,39). Although several centers are still utilizing conventional internal jugular-femoral or femoro-femoral VV configurations, more experienced centers now prefer DLC due to lesser rates of recirculation and opportunities to ambulation/rehabilitation with the DLC (40-43). In patients with a predominantly hypercapnic respiratory failure, lower ECMO flow rates may be as effective and hence a small caliber DLC or peripheral venous cannula can be utilized (44,45). However, patients with pre-existing significant primary/secondary pulmonary hypertension and/or right ventricular failure may potentially have worsening of their RV dysfunction with a VV configuration alone due to the increased venous return. In these circumstances, an atrial septostomy with VV (46,47) (either DLC or peripheral VV), peripheral VA or a central VA approach (48-50) (Table 2) may be considered. Based on individual patient requirements for additional ECMO blood flow or cardiac support, a mixed configuration with additional cannulation on the venous or arterial side can be performed. More recently, our group has utilized a directional catheter (double lumen VV cannula directed to pulmonary artery- Protek Duo™) approach successfully in patients with significant pulmonary hypertension (51). This approach can potentially reduce the need for further invasive procedures such as atrial septostomy or sternotomy for central cannulation in patients with pulmonary hypertension requiring ECMO support.
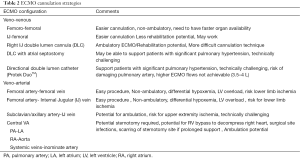
Full table
Management of pre-transplant patient on ECMO
Ventilator & sedation management
One of the primary objectives of ECMO support is to minimize the intensity and/or dependence on mechanical ventilation. After ECMO initiation, an effort should be made to reduce intensity of mechanical ventilation by reduction of PEEP and/or set Fio2 on the ventilator. Although, significant improvement in lung function cannot be expected in patients who are bridged to lung transplant on ECMO, lung protective ventilation strategies (tidal volume (6 cc/kg), plateau pressures <30 mmHg) should be adhered to minimize barotrauma and volutrauma related complications (52). In several cases, awake and cooperative patients who can tolerate weaning off mechanical ventilation can be safely extubated on ECMO support (53,54). In patients with ongoing ventilator need, an early tracheostomy should be preferred especially in those with increased secretions and/or intensive sedation requirements.
In our experience, an early tracheostomy facilitates sedative weaning and early ambulation. Attempts should be made to wean off sedation as tolerated. Although, sedation management is unique to each center, we prefer to avoid benzodiazepines and high dose narcotics.
Anticoagulation and blood transfusion management
Systemic anticoagulation remains an important component in ECMO management to maintain a thrombus-free circuit. Clots can develop in the oxygenator membrane and can prevent optimal gas exchange but rarely result in embolization to the systemic circulation. The goal of anticoagulation is to balance therapeutic doses to prevent circuit thrombus with minimization of bleeding complications. Unfortunately, bleeding remains a major complication of ECMO support with a cumulative incidence of up to 43% (22). Apart from the anticoagulation several other factors including the circuit related factors such as increased inlet and outlet pressures and kinks (leading to mechanical trauma to blood components), surgical procedures, and infections contribute to the risk of bleeding on ECMO (55). Minor bleeding episodes such as oozing from cannula or tracheostomy sites can be efficiently managed with compression dressings and/or instillation of subcutaneous vasoconstrictors. However, significant or catastrophic bleeding in the GI tract, central nervous system and the lungs have to be managed with cessation or sometimes reversal of anticoagulation. Unfractionated heparin is the most utilized anticoagulant for systemic anticoagulation on ECMO due to its shorter half-life and availability of a reversal agent in case of catastrophic bleeds. However, other agents such as bivalirudin and argatroban have also been used for anticoagulation on ECMO (56-58). Anticoagulation is usually tailored based on presence of clots in the circuit, coagulopathy and bleeding while on ECMO (59). Several groups have also reported reduced or no systemic anticoagulation use especially on VV ECMO support successfully (60,61). In our experience, if necessitated, it may be feasible to restrict anticoagulation in VV configuration in specific cases, but particular care should be taken to rule out presence of atrial/ventricular septal defects to prevent systemic embolism. In addition, careful circuit/oxygenator monitoring and use of heparin-bonded circuits with minimal adaptors/connections may be helpful in preventing excessive clot formation (62). In patients on a VA configuration, systemic anticoagulation should be maintained as tolerated due to risk of systemic embolism (63).
In the current era, sensitive assays are available to monitor anticoagulation status. The choice of anticoagulation monitoring tests is usually based on institutional practices. Activated clotting time (ACT), activated partial thromboplastin time (APTT), anti-factor Xa levels and thromboelastography (TEG) have all been utilized to measure level of anticoagulation on ECMO. Despite some limitations, recent studies describe the accuracy and ease of anti-factor Xa assays to monitor unfractionated heparin on ECMO (64,65). Additionally, TEG monitoring has also shown to be helpful in anticoagulation management on ECMO (66). Given the myriad of hematological changes occurring on ECMO due to both anticoagulation and ECMO circuit factors, it seems plausible that a protocol involving measurement of heparin activity with anti-factor Xa and overall clot reaction with TEG may be beneficial. At out institution, we utilize TEG monitoring and keep a reaction(R) time of 2.5–3 times normal (67). ELSO guidelines provide details about possible anticoagulation strategies (www.ELSO.org).
Another important aspect of ECMO management is the transfusion of blood products for bleeding complications and/or volume support. Traditionally, a hemoglobin level of 9–10 g/dL was suggested for optimal oxygenation and maintenance of circuit flow on ECMO (68). However, in pre-transplant patients, frequent blood product transfusion can lead to allo-sensitization which is associated with elevated panel reactive antibody (PRA) titers and production of donor related antibodies to blood products (69,70) that can potentially reduce donor allograft and recipient survival. Additionally, there is risk for transfusion associated reactions and complications from intravascular volume overload (71). Recent reports suggest that a more conservative transfusion strategy may lower transfusion requirements and bleeding complications, with comparable survival and organ recovery to a more liberal transfusion practice (72). During ECMO circuit changes due to any reason, auto transfusion of the patient’s blood in the circuit tubing can be performed using a cell-salvage device (HaemoneticsTM) to reduce the need for allo-transfusion (73). We suggest that blood product transfusion practices should be tailored to hemodynamic and oxygenation needs and a more conservative transfusion strategy should be followed to reduce allo-sensitization.
Ambulation on ECMO
Increased pre-transplant frailty is a risk factor for worse post lung transplant outcomes (74,75). Critically ill patients on prolonged mechanical ventilation and/or ECMO support are prone to developing significant neuro-muscular weakness (76,77). Several studies have demonstrated that physical rehabilitation in patients on ECMO support is safe and can potentially improve post-transplant recovery and outcomes (25,29,39,78,79). Ambulation can be safely achieved in patients cannulated via a DLC in a VV configuration or subclavian/central VA configuration (48,80-82). In patients with configurations requiring femoral cannulation, ambulation may result in cannula dislodgement and be potentially harmful (83). A multidisciplinary team comprising of a physician, nursing staff, perfusion, respiratory and physical therapists facilitates safe and effective physical rehabilitation on ECMO. At our center, patients bridged on ECMO ambulate several times a day as tolerated. Bedside upper and lower extremity strength training exercises are also performed regularly. In addition, in some cases, we have utilized activities such as video games on Wii (NintendoTM) to promote physical rehabilitation in our younger patients bridged on ECMO. Patients with prolonged ECMO support are also prone to severe mental health issues including major depression and post-traumatic stress disorder (37,84-86). Previous studies have shown that physical rehabilitation improves the mental health in several disease states (87). It is possible that it may help those supported with ECMO as well. In our experience, physical rehabilitation, and other recreational activities such as music therapy and creative art help patients cope with the daily stressors during ECMO support.
Perioperative management
Management of ECMO in the peri-operative period is largely based on individual surgeon preference. Several groups have reported continued utilization of ECMO support intra-operatively with reduced need for anticoagulation/blood transfusions, reduction in neurological and vascular complications (88-90). Others prefer to convert to a conventional cardiopulmonary bypass support intra-operatively. Continued post-operative ECMO in patients transplanted for pulmonary hypertension has been advocated by some to control reperfusion and provide non-aggressive ventilation after transplant (91). Additionally, ECMO support has been utilized for management of primary graft dysfunction after lung transplantation (36,37).
ECLS during organ procurement
There has been recent interest in providing extracorporeal membrane support to marginal/higher risk donor organs during organ harvesting (92). The use of this technology called the ex vivo lung perfusion (EVLP) allows transplantation of marginal/high-risk donor lungs that are physiologically stable during ex vivo perfusion. Recent reports have shown that post-operative outcomes in organs supported with EVLP technology is similar to those obtained with conventionally selected lungs (92-94).
Another application of ECLS technology in organ procurement is harvesting of lungs after donor cardiac death (DCD). This technology involves deployment of VA ECMO support in donors after cardiac death to continue organ perfusion and harvesting. Outcomes of DCD lung have been shown to be similar to conventionally harvested organs (95-97). Sometimes EVLP support is utilized after DCD organ harvesting and may result in improved outcomes (98). The overall long-term impact of EVLP and DCD organ donation in lung transplantation is not fully understood, and further research is needed to establish a clear role for these technologies in organ transplantation.
Conclusions
Despite limited data to support its use, there has been a recent increase in utilization of ECMO as a bridge to lung transplantation. ECMO as a bridge to transplantation is not suited for all patients. Careful patient selection should be performed to optimize resource utilization and provide the best opportunity for transplantation.
Acknowledgements
We would like to acknowledge Mr. Peter Rycus from the Extracorporeal Life Support Organization (ELSO) in providing the data for ECLS use in lung transplant patients.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- MacLaren G, Combes A, Bartlett RH. Contemporary extracorporeal membrane oxygenation for adult respiratory failure: life support in the new era. Intensive Care Med 2012;38:210-20. [Crossref] [PubMed]
- Gattinoni L, Kolobow T, Agostoni A, et al. Clinical application of low frequency positive pressure ventilation with extracorporeal CO2 removal (LFPPV-ECCO2R) in treatment of adult respiratory distress syndrome (ARDS). Int J Artif Organs 1979;2:282-3. [PubMed]
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193-6. [Crossref] [PubMed]
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;149:295-305. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009;302:1888-95. [Crossref] [PubMed]
- Chan KK, Lee KL, Lam PK, et al. Hong Kong's experience on the use of extracorporeal membrane oxygenation for the treatment of influenza A (H1N1). Hong Kong Med J 2010;16:447-54. [PubMed]
- Freed DH, Henzler D, White CW, et al. Extracorporeal lung support for patients who had severe respiratory failure secondary to influenza A (H1N1) 2009 infection in Canada. Can J Anaesth 2010;57:240-7. [Crossref] [PubMed]
- Nair P, Davies AR, Beca J, et al. Extracorporeal membrane oxygenation for severe ARDS in pregnant and postpartum women during the 2009 H1N1 pandemic. Intensive Care Med 2011;37:648-54. [Crossref] [PubMed]
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011;306:1659-68. [Crossref] [PubMed]
- Patroniti N, Zangrillo A, Pappalardo F, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med 2011;37:1447-57. [Crossref] [PubMed]
- Pham T, Combes A, Roze H, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome: a cohort study and propensity-matched analysis. Am J Respir Crit Care Med 2013;187:276-85. [Crossref] [PubMed]
- Turner DA, Rehder KJ, Peterson-Carmichael SL, et al. Extracorporeal membrane oxygenation for severe refractory respiratory failure secondary to 2009 H1N1 influenza A. Respir Care 2011;56:941-6. [Crossref] [PubMed]
- Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA 2009;302:1872-9. [Crossref] [PubMed]
- Sauer CM, Yuh DD, Bonde P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J 2015;61:31-6. [Crossref] [PubMed]
- Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant 2006;6:1212-27. [Crossref] [PubMed]
- Russo MJ, Worku B, Iribarne A, et al. Does lung allocation score maximize survival benefit from lung transplantation? J Thorac Cardiovasc Surg 2011;141:1270-77. [Crossref] [PubMed]
- Mason DP, Thuita L, Nowicki ER, et al. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg 2010;139:765-773.e1. [Crossref] [PubMed]
- George TJ, Beaty CA, Kilic A, et al. Outcomes and temporal trends among high-risk patients after lung transplantation in the United States. J Heart Lung Transplant 2012;31:1182-91. [Crossref] [PubMed]
- Hayanga JW, Lira A, Aboagye JK, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation: what lessons might we learn from volume and expertise? Interact Cardiovasc Thorac Surg 2016;22:406-10. [Crossref] [PubMed]
- Hayanga AJ, Aboagye J, Esper S, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation in the United States: an evolving strategy in the management of rapidly advancing pulmonary disease. J Thorac Cardiovasc Surg 2015;149:291-6. [Crossref] [PubMed]
- Extracorporeal Life Support Organization. ECLS Registry report 2016. Available online: http://www.elso.org/
- Weiss ES, Allen JG, Meguid RA, et al. The impact of center volume on survival in lung transplantation: an analysis of more than 10,000 cases. Ann Thorac Surg 2009;88:1062-70. [Crossref] [PubMed]
- Hayes D Jr, Tobias JD, Tumin D. Center Volume and Extracorporeal Membrane Oxygenation Support at Lung Transplantation in the Lung Allocation Score Era. Am J Respir Crit Care Med 2016;194:317-26. [Crossref] [PubMed]
- Hoopes CW, Kukreja J, Golden J, et al. Extracorporeal membrane oxygenation as a bridge to pulmonary transplantation. J Thorac Cardiovasc Surg 2013;145:862-7; discussion 867-868. [Crossref] [PubMed]
- Shafii AE, Mason DP, Brown CR, et al. Growing experience with extracorporeal membrane oxygenation as a bridge to lung transplantation. ASAIO J 2012;58:526-9. [Crossref] [PubMed]
- Hämmäinen P, Schersten H, Lemström K, et al. Usefulness of extracorporeal membrane oxygenation as a bridge to lung transplantation: a descriptive study. J Heart Lung Transplant 2011;30:103-7. [Crossref] [PubMed]
- Javidfar J, Brodie D, Iribarne A, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation and recovery. J Thorac Cardiovasc Surg 2012;144:716-21. [Crossref] [PubMed]
- Rehder KJ, Turner DA, Hartwig MG, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care 2013;58:1291-8. [Crossref] [PubMed]
- Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant 2016;35:433-9. [Crossref] [PubMed]
- Russo MJ, Meltzer D, Merlo A, et al. Local allocation of lung donors results in transplanting lungs in lower priority transplant recipients. Ann Thorac Surg 2013;95:1231-4; discussion 1234-5. [Crossref] [PubMed]
- Salam S, Kotloff R, Garcha P, et al. Lung transplanation after 125 days on ECMO for severe refractory hypoxemia with no prior lung disease. ASAIO J 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Tissot C, Habre W, Soccal P, et al. Successful Lung Transplant After Prolonged Extracorporeal Membrane Oxygenation (ECMO) in a Child With Pulmonary Hypertension: A Case Report. Res Cardiovasc Med 2016;5:e32545. [Crossref] [PubMed]
- Broomé M, Palmér K, Scherstén H, et al. Prolonged extracorporeal membrane oxygenation and circulatory support as bridge to lung transplant. Ann Thorac Surg 2008;86:1357-60. [Crossref] [PubMed]
- Kon ZN, Wehman PB, Gibber M, et al. Venovenous extracorporeal membrane oxygenation as a bridge to lung transplantation: successful transplantation after 155 days of support. Ann Thorac Surg 2015;99:704-7. [Crossref] [PubMed]
- Glassman LR, Keenan RJ, Fabrizio MC, et al. Extracorporeal membrane oxygenation as an adjunct treatment for primary graft failure in adult lung transplant recipients. J Thorac Cardiovasc Surg 1995;110:723-6; discussion 726-7. [Crossref] [PubMed]
- Zenati M, Pham SM, Keenan RJ, et al. Extracorporeal membrane oxygenation for lung transplant recipients with primary severe donor lung dysfunction. Transpl Int 1996;9:227-30. [Crossref] [PubMed]
- Corso PJ, Geelhoed GW, Joseph WL. Membrane lung oxygenation for temporary support of the failing transplanted lung. Trans Am Soc Artif Intern Organs 1973;19:525-8. [Crossref] [PubMed]
- Turner DA, Cheifetz IM, Rehder KJ, et al. Active rehabilitation and physical therapy during extracorporeal membrane oxygenation while awaiting lung transplantation: a practical approach. Crit Care Med 2011;39:2593-8. [Crossref] [PubMed]
- Kuhl T, Michels G, Pfister R, et al. Comparison of the Avalon Dual-Lumen Cannula with Conventional Cannulation Technique for Venovenous Extracorporeal Membrane Oxygenation. Thorac Cardiovasc Surg 2015;63:653-62. [Crossref] [PubMed]
- Vaja R, Chauhan I, Joshi V, et al. Five-year experience with mobile adult extracorporeal membrane oxygenation in a tertiary referral center. J Crit Care 2015;30:1195-8. [Crossref] [PubMed]
- De Bartolo C, Nigro A, Fragomeni G, et al. Numerical and experimental flow analysis of the Wang-Zwische double-lumen cannula. ASAIO J 2011;57:318-27. [Crossref] [PubMed]
- Wang D, Zhou X, Liu X, et al. Wang-Zwische double lumen cannula-toward a percutaneous and ambulatory paracorporeal artificial lung. ASAIO J 2008;54:606-11. [Crossref] [PubMed]
- Abrams DC, Brenner K, Burkart KM, et al. Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013;10:307-14. [Crossref] [PubMed]
- Tiruvoipati R, Buscher H, Winearls J, et al. Early experience of a new extracorporeal carbon dioxide removal device for acute hypercapnic respiratory failure. Crit Care Resusc 2016;18:261-9. [PubMed]
- Kon ZN, Pasrija C, Shah A, et al. Venovenous Extracorporeal Membrane Oxygenation With Atrial Septostomy as a Bridge to Lung Transplantation. Ann Thorac Surg 2016;101:1166-9. [Crossref] [PubMed]
- Camboni D, Akay B, Sassalos P, et al. Use of venovenous extracorporeal membrane oxygenation and an atrial septostomy for pulmonary and right ventricular failure. Ann Thorac Surg 2011;91:144-9. [Crossref] [PubMed]
- Chicotka S, Rosenzweig EB, Brodie D, et al. The "Central Sport Model": Extracorporeal Membrane Oxygenation Using the Innominate Artery for Smaller Patients as Bridge to Lung Transplantation. ASAIO J 2016. [Epub ahead of print].
- Saeed D, Stosik H, Islamovic M, et al. Femoro-femoral versus atrio-aortic extracorporeal membrane oxygenation: selecting the ideal cannulation technique. Artif Organs 2014;38:549-55. [Crossref] [PubMed]
- Saeed D, Maxhera B, Westenfeld R, et al. An Alternative Approach for Perioperative Extracorporeal Life Support Implantation. Artif Organs 2015;39:719-23. [Crossref] [PubMed]
- Diaz-Guzman E, Sharma NS, Wille K, et al. Use of a novel pulmonary artery cannula to provide extracorporeal membrane oxygenation as a bridge to lung transplantation. J Heart Lung Transplant 2016;35:1051-3. [Crossref] [PubMed]
- Klompas M. Complications of mechanical ventilation--the CDC's new surveillance paradigm. N Engl J Med 2013;368:1472-5. [Crossref] [PubMed]
- Anton-Martin P, Thompson MT, Sheeran PD, et al. Extubation during pediatric extracorporeal membrane oxygenation: a single-center experience. Pediatr Crit Care Med 2014;15:861-9. [Crossref] [PubMed]
- Bein T, Wittmann S, Philipp A, et al. Successful extubation of an "unweanable" patient with severe ankylosing spondylitis (Bechterew's disease) using a pumpless extracorporeal lung assist. Intensive Care Med 2008;34:2313-4. [Crossref] [PubMed]
- Aubron C, DePuydt J, Belon F, et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann Intensive Care 2016;6:97. [Crossref] [PubMed]
- Pieri M, Agracheva N, Bonaveglio E, et al. Bivalirudin versus heparin as an anticoagulant during extracorporeal membrane oxygenation: a case-control study. J Cardiothorac Vasc Anesth 2013;27:30-4. [Crossref] [PubMed]
- Young G, Yonekawa KE, Nakagawa P, et al. Argatroban as an alternative to heparin in extracorporeal membrane oxygenation circuits. Perfusion 2004;19:283-8. [Crossref] [PubMed]
- Johnston N, Wait M, Huber L. Argatroban in adult extracorporeal membrane oxygenation. J Extra Corpor Technol 2002;34:281-4. [PubMed]
- Bembea MM, Schwartz JM, Shah N, et al. Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J 2013;59:63-8. [Crossref] [PubMed]
- Krueger K, Schmutz A, Zieger B, et al. Venovenous Extracorporeal Membrane Oxygenation With Prophylactic Subcutaneous Anticoagulation Only: An Observational Study in More Than 60 Patients. Artif Organs 2017;41:186-92. [Crossref] [PubMed]
- Sklar MC, Sy E, Lequier L, et al. Anticoagulation Practices during Venovenous Extracorporeal Membrane Oxygenation for Respiratory Failure. A Systematic Review. Ann Am Thorac Soc 2016;13:2242-50. [Crossref] [PubMed]
- Hastings SM, Ku DN, Wagoner S, et al. Sources of Circuit Thrombosis in Pediatric Extracorporeal Membrane Oxygenation. ASAIO J 2017;63:86-92. [Crossref] [PubMed]
- Lafç G, Budak AB, Yener AÜ, et al. Use of extracorporeal membrane oxygenation in adults. Heart Lung Circ 2014;23:10-23. [Crossref] [PubMed]
- O'Meara LC, Alten JA, Goldberg KG, et al. Anti-xa directed protocol for anticoagulation management in children supported with extracorporeal membrane oxygenation. ASAIO J 2015;61:339-44. [Crossref] [PubMed]
- Irby K, Swearingen C, Byrnes J, et al. Unfractionated heparin activity measured by anti-factor Xa levels is associated with the need for extracorporeal membrane oxygenation circuit/membrane oxygenator change: a retrospective pediatric study. Pediatr Crit Care Med 2014;15:e175-82. [Crossref] [PubMed]
- Stammers AH, Willett L, Fristoe L, et al. Coagulation monitoring during extracorporeal membrane oxygenation: the role of thrombelastography. J Extra Corpor Technol 1995;27:137-45. [PubMed]
- Sharma NS, Wille KM, Bellot SC, et al. Modern use of extracorporeal life support in pregnancy and postpartum. ASAIO J 2015;61:110-4. [Crossref] [PubMed]
- Annich G, Lynch W, MacLaren G, et al. ECMO: Extracorporeal Cardiopulmonary Support in Critical Care 4th Edition. Available online: https://www.elso.org/Publications/RedBook4thEdition.aspx
- Lam CP, Chow MP. HLA antibodies in multiple transfused patients. Zhonghua Yi Xue Za Zhi (Taipei) 1992;50:439-42. [PubMed]
- Hayes D Jr, Preston TJ, Kirkby S, et al. Human leukocyte antigen sensitization in lung transplant candidates supported by extracorporeal membrane oxygenation. Am J Respir Crit Care Med 2013;188:627-8. [Crossref] [PubMed]
- Smith A, Hardison D, Bridges B, et al. Red blood cell transfusion volume and mortality among patients receiving extracorporeal membrane oxygenation. Perfusion 2013;28:54-60. [Crossref] [PubMed]
- Agerstrand CL, Burkart KM, Abrams DC, et al. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg 2015;99:590-5. [Crossref] [PubMed]
- Preston TJ, Olshove VF Jr, Chase M. Bloodless extracorporeal membrane oxygenation in the Jehovah's Witness patient. J Extra Corpor Technol 2012;44:39-42. [PubMed]
- Singer JP, Diamond JM, Gries CJ, et al. Frailty Phenotypes, Disability, and Outcomes in Adult Candidates for Lung Transplantation. Am J Respir Crit Care Med 2015;192:1325-34. [Crossref] [PubMed]
- Maury G, Langer D, Verleden G, et al. Skeletal muscle force and functional exercise tolerance before and after lung transplantation: a cohort study. Am J Transplant 2008;8:1275-81. [Crossref] [PubMed]
- De Jonghe B, Sharshar T, Lefaucheur JP, et al. Paresis acquired in the intensive care unit: a prospective multicenter study. JAMA 2002;288:2859-67. [Crossref] [PubMed]
- Ali NA, O'Brien JM Jr, Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med 2008;178:261-8. [Crossref] [PubMed]
- Garcia JP, Iacono A, Kon ZN, et al. Ambulatory extracorporeal membrane oxygenation: a new approach for bridge-to-lung transplantation. J Thorac Cardiovasc Surg 2010;139:e137-9. [Crossref] [PubMed]
- Bain JC, Turner DA, Rehder KJ, et al. Economic Outcomes of Extracorporeal Membrane Oxygenation With and Without Ambulation as a Bridge to Lung Transplantation. Respir Care 2016;61:1-7. [Crossref] [PubMed]
- Biscotti M, Bacchetta M. The "sport model": extracorporeal membrane oxygenation using the subclavian artery. Ann Thorac Surg 2014;98:1487-9. [Crossref] [PubMed]
- Bermudez CA, Rocha RV, Zaldonis D, et al. Extracorporeal membrane oxygenation as a bridge to lung transplant: midterm outcomes. Ann Thorac Surg 2011;92:1226-31; discussion 1231-2. [Crossref] [PubMed]
- Hayanga JW, Aboagye JK, Hayanga HK, et al. Extracorporeal membrane oxygenation as a bridge to lung re-transplantation: Is there a role? J Heart Lung Transplant 2016;35:901-5. [Crossref] [PubMed]
- Rajagopal K, Hoeper MM. State of the Art: Bridging to lung transplantation using artificial organ support technologies. J Heart Lung Transplant 2016;35:1385-98. [Crossref] [PubMed]
- Tramm R, Ilic D, Murphy K, et al. A qualitative exploration of acute care and psychological distress experiences of ECMO survivors. Heart Lung 2016;45:220-6. [Crossref] [PubMed]
- Tramm R, Hodgson C, Ilic D, et al. Identification and prevalence of PTSD risk factors in ECMO patients: A single centre study. Aust Crit Care 2015;28:31-6. [Crossref] [PubMed]
- Hodgson CL, Hayes K, Everard T, et al. Long-term quality of life in patients with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation for refractory hypoxaemia. Crit Care 2012;16:R202. [Crossref] [PubMed]
- Hopkins RO, Suchyta MR, Farrer TJ, et al. Improving post-intensive care unit neuropsychiatric outcomes: understanding cognitive effects of physical activity. Am J Respir Crit Care Med 2012;186:1220-8. [Crossref] [PubMed]
- Machuca TN, Collaud S, Mercier O, et al. Outcomes of intraoperative extracorporeal membrane oxygenation versus cardiopulmonary bypass for lung transplantation. J Thorac Cardiovasc Surg 2015;149:1152-7. [Crossref] [PubMed]
- Hoechter DJ, von Dossow V, Winter H, et al. The Munich Lung Transplant Group. Intraoperative Extracorporeal Circulation in Lung Transplantation. Thorac Cardiovasc Surg 2015;63:706-14. [Crossref] [PubMed]
- Ius F, Sommer W, Tudorache I, et al. Five-year experience with intraoperative extracorporeal membrane oxygenation in lung transplantation: Indications and midterm results. J Heart Lung Transplant 2016;35:49-58. [Crossref] [PubMed]
- Pereszlenyi A, Lang G, Steltzer H, et al. Bilateral lung transplantation with intra- and postoperatively prolonged ECMO support in patients with pulmonary hypertension. Eur J Cardiothorac Surg 2002;21:858-63. [Crossref] [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [Crossref] [PubMed]
- Sanchez PG, Davis RD, D’Ovidio F, et al. The NOVEL Lung Trial One-Year Outcomes. The Journal of Heart and Lung Transplantation 2014;33:S71-2. [Crossref]
- Warnecke G, Van Raemdonck D, Massard G, et al. The INSPIRE Lung International Trial Evaluating the Impact of Portable Ex-vivo Perfusion Using the Organ Care System (OCS™) Lung Technology on Routine Lung Transplant Outcomes. J Heart Lung Transplant 33:S72. [Crossref]
- Cypel M, Levvey B, Van Raemdonck D, et al. International Society for Heart and Lung Transplantation Donation After Circulatory Death Registry Report. J Heart Lung Transplant 2015;34:1278-82. [Crossref] [PubMed]
- De Oliveira NC, Osaki S, Maloney JD, et al. Lung transplantation with donation after cardiac death donors: long-term follow-up in a single center. J Thorac Cardiovasc Surg 2010;139:1306-15. [Crossref] [PubMed]
- Sabashnikov A, Patil NP, Popov AF, et al. Long-term results after lung transplantation using organs from circulatory death donors: a propensity score-matched analysisdagger. Eur J Cardiothorac Surg 2016;49:46-53. [Crossref] [PubMed]
- Machuca TN, Mercier O, Collaud S, et al. Lung transplantation with donation after circulatory determination of death donors and the impact of ex vivo lung perfusion. Am J Transplant 2015;15:993-1002. [Crossref] [PubMed]


