A brother and sister with breast cancer, BRCA2 mutations and bilateral supernumerary nipples
Introduction
Supernumerary nipples are congenital malformations of nipples or their related tissue that arise in addition to the normal bilateral nipples of the chest. Their prevalence is between 0.22% and 5.6% (1), although there may be under-reporting. Supernumerary nipples typically appear along the embryonic milk lines from axillae to inguinal regions. Ectopic supernumerary nipples may be found beyond the milk lines. Supernumerary nipples may arise sporadically or through familial inheritance via autosomal dominant, X-linked dominant or X-linked recessive transmission (1-3). Their main significance lies in the potential for malignancies to arise in underlying breast tissue, especially in ectopic locations (4) and, in the association between supernumerary nipples and genitourinary malformations and malignancies (5).
Case presentation
A phenotypically normal 54-year-old man was diagnosed with infiltrating ductal carcinoma of the right breast for which he underwent right mastectomy and right axillary clearance. Seven of ten right axillary lymph nodes were positive for metastatic disease. His histopathological staging was pT1pN2M0.
He received postoperative chemotherapy and radiotherapy to the right chest wall and right supraclavicular fossa. He was subsequently commenced on tamoxifen.
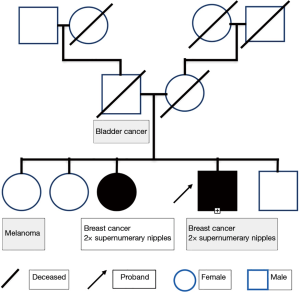
There was no evidence of cancer recurrence at follow up 12 months after completing radiotherapy. However, at the follow up examination he was incidentally noted to have bilateral supernumerary nipples along the milk line on the lower chest/upper abdomen (Figure 1). Clinically, the patient or his relatives had no genetic syndrome. However, his family history was significant for one sister having had breast cancer diagnosed at age 40 (Figure 2). Interestingly, the same sister also had bilateral supernumerary nipples, which had previously been removed for cosmetic reasons, and a BRCA2 mutation, as had our case.
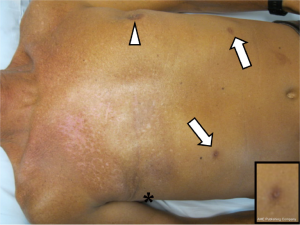
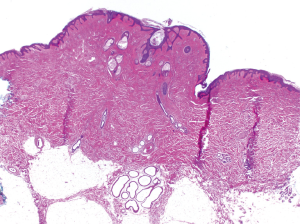
Ultrasound in the region of the supernumerary nipples revealed elevation of the skin with a little underlying hypoechoic tissue. No convincing mass was seen and there was no connection to deeper structures. The soft tissues of the anterior abdominal wall appeared normal. We recommended removal of the two supernumerary nipples, which were subsequently resected. The macroscopic and microscopic appearance of the nipples was normal. The histological appearance of the resected specimen is shown in Figure 3.
Discussion
Supernumerary nipples most commonly arise along the milk lines, which extend from the axilla to the groin bilaterally. However, they may also appear in other regions of the chest and abdomen, as well as the thighs, upper limbs, posterior thorax and head (4,6,7). Supernumerary nipples may be associated with underlying breast tissue. This breast tissue can have many, if not all, the histologic characteristics of normal breast tissue. As a result, breast adenomas and malignancies can arise in the breast tissue underlying supernumerary nipples. Furthermore, ectopic breast tissue is more prone to malignant change than normal breast tissue. Evidence also exists that ectopic breast cancer carries a poorer prognosis than cancer arising in normal breast tissue; recommendations have been made for regular cancer screening in ectopic breast tissue and consideration of prophylactic resection (8,9).
Of further significance is the association between supernumerary nipples and genitourinary tract cancer, as well as possibly increased genitourinary tract malformations (10). As a result, our patient underwent ultrasound of the genitourinary tract. The ultrasound findings were normal.
Our patient’s family tree is consistent with an autosomal dominant pattern of breast cancer inheritance, a mode characteristic of BRCA2 mutations and which has also been reported previously for supernumerary nipples (10). The pattern would also be consistent with a X-linked pattern of inheritance with variable penetrance. There are a number of syndromes associated with supernumerary nipples. Those with known gene mutations are summarised in Table 1. Supernumerary nipples have also been associated with syndromes due to aneuploidy or partial aneuploidy and include Turner syndrome, trisomy 8, partial chromosome 3p trisomy (10) and trisomy 2p syndrome (11). Fleisher’s syndrome is another very rare syndrome associated with supernumerary nipples (10).

Full table
Male breast cancer accounts for about 1% of all incidents of breast carcinoma. Approximately 20% of men with breast cancer have a family history of the disease. Genetic risk factors for breast cancer in men include Klinefelter syndrome and BRCA1 and BRCA2 mutations, with the latter a more characteristic predisposing factor. Mutations in the PTEN tumour suppressor gene and the mismatch repair gene human mutL homolog 1 (hMLH1) have been associated with, but not clearly shown, to increase breast cancer risk in men (25).
Conclusions
The kindred we describe here carries the breast cancer predisposition gene, BRCA2. It is anyway a ‘high risk’ kindred for having a BRCA1 or BRCA2 mutation, in that one female had breast cancer at an early age and there was a breast cancer-affected male in the kindred. Given the rarity of male breast cancer and supernumerary nipples, the possibility exists that a mutation in the BRCA2 gene affected its associated functions and is responsible for both the breast cancer and the supernumerary nipples. Should this be the case, wild-type BRCA2 would be acting as a suppressor of supernumerary nipple formation.
Given the risk of breast cancer arising in ectopic breast tissue we believe that consideration should be given to removal of supernumerary nipples and any associated breast tissue, in patients previously diagnosed with breast cancer and in patients for which there is a family history of breast cancer.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics approval for this study was given by North Coast Area Health Region Ethics Committee (ID: 08-16) and the patient who was the subject of the case report gave consent for reproduction of a de-identified photograph of himself.
References
- Galli-Tsinopoulou A, Krohn C, Schmidt H. Familial polythelia over three generations with polymastia in the youngest girl. Eur J Pediatr 2001;160:375-7. [Crossref] [PubMed]
- Toumbis-Ioannou E, Cohen PR. Familial polythelia. J Am Acad Dermatol 1994;30:667-8. [Crossref] [PubMed]
- Mehes K. Familial association of supernumerary nipple with renal cancer. Cancer Genet Cytogenet 1996;86:129-30. [Crossref] [PubMed]
- Johnson CA, Felson B, Jolles H. Polythelia (supernumerary nipple): an update. South Med J 1986;79:1106-8. [Crossref] [PubMed]
- Casey HD, Chasan PE, Chick LR. Familial polythelia without associated anomalies. Ann Plast Surg 1996;36:101-4. [Crossref] [PubMed]
- Camisa C. Accessory breast on the posterior thigh of a man. J Am Acad Dermatol 1980;3:467-9. [Crossref] [PubMed]
- Hanson E, Segóvia J. Dorsal supernumerary breast. Case report. Plast Reconstr Surg 1978;61:441-5. [Crossref] [PubMed]
- Roorda AK, Hansen JP, Rider JA, et al. Ectopic breast cancer: special treatment considerations in the postmenopausal patient. Breast J 2002;8:286-9. [Crossref] [PubMed]
- Sharma A, Sharma M, Sharma V, et al. Lactating adenoma of ectopic breast tissue in the vulvar region. Indian Journal of Basic and Applied Medical Research 2013;3:212-4.
- Grimshaw EC, Cohen PR. Supernumerary nipple and seminoma: case report and review of polythelia and genitourinary cancers. Dermatol Online J 2013;19:4. [PubMed]
- Gómez-Raposo C, Zambrana Tévar F, Sereno Moyano M, et al. Male breast cancer. Cancer Treat Rev 2010;36:451-7. [Crossref] [PubMed]
- Yilmaz AE, Sarifakioglu E, Dogan G, et al. Supernumerary nipple: should we be alert? Pediatr Int 2010;52:e190-1. [Crossref] [PubMed]
- Hu Z, Bonifas JM, Beech J, et al. Mutations in ATP2C1, encoding a calcium pump, cause Hailey-Hailey disease. Nat Genet 2000;24:61-5. [Crossref] [PubMed]
- Kalay E, Sezgin O, Chellappa V, et al. Mutations in RIPK4 cause the autosomal-recessive form of popliteal pterygium syndrome. Am J Hum Genet 2012;90:76-85. [Crossref] [PubMed]
- Satoda M, Zhao F, Diaz GA, et al. Mutations in TFAP2B cause Char syndrome, a familial form of patent ductus arteriosus. Nat Genet 2000;25:42-6. [Crossref] [PubMed]
- Anjankar SD, Subodh R. Spondylocostal dysostosis with lipomyelomeningocele: Case report and review of the literature. J Pediatr Neurosci 2014;9:249-52. [Crossref] [PubMed]
- Yilmaz MB, Kaymak A, Kurt G, et al. Spondylocostal dysostosis associated with type I split cord malformation and double nipple on one side: a case report. Turk Neurosurg 2013;23:256-9. [PubMed]
- Duru S, Ceylan S, Güvenç BH. Segmental costovertebral malformations: association with neural tube defects. Report of 3 cases and review of the literature. Pediatr Neurosurg 1999;30:272-7. [Crossref] [PubMed]
- Pilia G, Hughes-Benzie RM, MacKenzie A, et al. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet 1996;12:241-7. [Crossref] [PubMed]
- Filmus J, Capurro M, Rast J. Glypicans. Genome Biol 2008;9:224. [Crossref] [PubMed]
- Trovó-Marqui AB, Tajara EH. Neurofibromin: a general outlook. Clin Genet 2006;70:1-13. [Crossref] [PubMed]
- Mitchell K, O'Sullivan J, Missero C, et al. Exome sequence identifies RIPK4 as the Bartsocas-Papas syndrome locus. Am J Hum Genet 2012;90:69-75. [Crossref] [PubMed]
- Williamson JA, Bosher JM, Skinner A, et al. Chromosomal mapping of the human and mouse homologues of two new members of the AP-2 family of transcription factors. Genomics 1996;35:262-4. [Crossref] [PubMed]
- Shen MH, Harper PS, Upadhyaya M. Molecular genetics of neurofibromatosis type 1 (NF1). J Med Genet 1996;33:2-17. [Crossref] [PubMed]
- Ton VK, Mandal D, Vahadji C, et al. Functional expression in yeast of the human secretory pathway Ca(2+), Mn(2+)-ATPase defective in Hailey-Hailey disease. J Biol Chem 2002;277:6422-7. [Crossref] [PubMed]