Is the SenseWear Armband accurate enough to quantify and estimate energy expenditure in healthy adults?
Introduction
In recent years, there has been an increase in experimental and epidemiological studies confirming the connection between physical activity (PA), health parameters, and global mortality in different populations (1). It has been shown that people who perform PA on a regular basis are less likely to suffer from chronic illness or premature death (2). The health benefits of PA also have repercussions on a national economic level as health costs for physically active people are lower (3). Because of the importance of this relationship, very accurate PA measurement methods are needed to establish more specific links between PA and health outcomes and to evaluate the effectiveness of intervention programs (4).
Although there are methods that are considered to be gold standards for the validation of other methods, such as direct calorimetry, doubly labelled water (DLW), indirect calorimetry (IC), and direct observation (5), accelerometers are the most commonly used monitors for research and medical purposes when quantifying levels of PA in subjects (6). They estimate the movement of the body based on changes in acceleration over time on one, two, or three planes, depending on the characteristics of the accelerometer used (7). To obtain good measurements, they must be worn very close to the body and as near as possible to the center of gravity (7). To measure the acceleration intensity, accelerometers use a piezoelectric sensor (“cantilever beam” or “integrated chip sensor”) and microprocessors (8). However, the need for improvement has led to the recent emergence of a new technology that combines the accelerometer data with other physiological sensors integrated into a single device, such as the SenseWear Armband (SWA) (BodyMedia Inc., Pittsburgh, PA).
The SWA is a relatively new device that can be used to quantify energy expenditure (EE) and is designed to be worn on the upper arm over the triceps. Its internal sensors include an accelerometer, a thermal flow sensor, a galvanic sensor that records skin response, a skin temperature sensor, and an air temperature sensor (9). The accelerometer in the armband has two axes and uses a microelectromechanical sensor that measures movement. The software created by the manufacturer calculates EE using a patented algorithm that combines acceleration, heat flow, and other parameters. However, the percentage of each parameter (>20 output parameters in total) that contributes to the prediction equation is unclear (10).
The SWA has the advantage of being able to quantify EE for very low-intensity activities or during static exercises that do not require walking or running (9). It is for this reason that it has been chosen for EE quantification studies in individuals with low mobility, such as older individuals [no difference in mean ± standard deviation (SD) values between the DLW and SWA methods, P=0.59; intraclass correlation coefficient, ICC =0.87] (11), women with rheumatoid arthritis (ICC for 7 days =0.96) (12), and people with hemiplegia (ICC =0.59 for the hemiplegic arm, and ICC =0.70 for the unaffected arm) (9). The SWA has also been validated in young adults (nonsignificant differences versus IC in mean ± SD) and sedentary people (moderate correlation with IC, r =0.47–0.69) for different levels of PA (13,14). However, to the best of our knowledge, no validation studies have been conducted in healthy adults. Thus, the aim of the present study was to evaluate the precision and validity of the SWA monitor for quantification of PA and EE levels in healthy adults aged between 40 and 55 years.
Methods
Participants
Twenty-three healthy adults (56.5% women) aged between 40 and 55 years participated in the study. The anthropometric characteristics of the participants were as follows: age (48±3.42 years), height (167.0±10.73 cm), body mass (63±13.35 kg), body mass index (22.78±6.38%), and muscle mass (48.25±9.53 kg), the latter of which was estimated using electrical bioimpedance (electrical bioimpedance; Tanita BC 420SMA Portable Body Composition Monitor).
The protocol was developed in accordance with the Helsinki Declaration of 1964 for research involving humans (considering the latest modification of 2013), the study was approved by the University’s Human Ethics Committee (University of Zaragoza), and all participants signed an informed consent form for participation in the study. The participants were excluded from the study if they were suffering from a musculoskeletal or cardiovascular disease that would impede them from performing the set protocols, if they were under the effects of any medication that may alter their metabolism, and if they had any other contraindication for PA. All participants completed a physical activity readiness questionnaire (PAR-Q) prior to performing the experimental protocol. Only two participants were excluded from the study because they answered “yes” to one or more of the questions on the questionnaire.
Experimental protocol
All participants performed the following standardized PA for 10 minutes each, with 5 minutes of rest between each activity: remaining at rest, walking at 3 km·h-1, walking at 5 km·h-1, running at 7 km·h-1, running at 9 km·h-1, and sitting and standing with a chair at a rate of 30 cycle·min-1 using a metronome for accuracy. The treadmill was set at an incline of 0° for all activities.
The participants wore the SWA on their right arms throughout the protocol, and their EE level was measured simultaneously using an indirect calorimeter.
Materials
A model Quasar Med 4.0 h/p/cosmos treadmill was used (Nussdorf-Traunstein, Germany).
The indirect calorimeter was used to measure EE and oxygen consumption for each activity using open circuit ergospirometry with an Oxycon Pro metabolic measurement system from Jaeger-Viasys Healthcare (Hoechberg, Germany), which provides breath-by-breath gas analysis. Occasional non-standard respirations (e.g., coughing, speaking, or sneezing) were eliminated from the dataset when a value exceeded three times the SD from the mean, which was calculated using the average of two intervals before and two intervals after the non-standard respirations (15). The volume of the gas analyzer was calibrated on a daily basis using a gas with known content levels (16% O2 and 5% CO2) (16).
The SWA was initiated via a USB-PC connection using the software provided by the manufacturer and was synchronized with the metabolic measurement system. The variables of gender, age, weight, and height obtained through electrical bioimpedance for each participant were entered prior to carrying out each test.
Statistical analysis
Of the 10 minutes over which measurements were taken, only the middle 5 were selected for data analysis for each activity and for each of the data collection methods.
To identify any significant differences between the caloric expenditure estimation methods (indirect calorimeter/SWA) for each of the standardized PAs (rest, walking at 3 and 5 km·h-1, running at 7 and 9 km·h-1, and sitting/standing with a chair), an analysis of variance (ANOVA) was conducted using the measurements repeated 2×6 times. If significant differences were found, the Bonferroni test was used as a post-hoc test to compare pairs. Bland-Altman analysis was also conducted to evaluate concordance between the two EE estimation methods. Calculations were made of the values of the differences in means (BIAS), SD, and limits of agreement at 95% (LOA). The association between the difference in means and the magnitude of the measurements (e.g., heteroscedasticity) was also examined via regression analysis. For the latter analysis, the difference between the SWA and indirect calorimeter values was introduced as a dependent variable, while the mean value [(SWA METs + indirect calorimeter METs)/2] was defined as the independent variable for each activity.
Finally, the sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve (AUC) were calculated to evaluate the precision of the SWA in classifying different levels of PA. The maximum value of the AUC is 1 and represents perfect classification; a value of 0.50 indicates the complete absence of accuracy in the classification. It is considered that values ≥0.90 are excellent, ≥0.80–0.90 are good, 0.8–0.70 are average, and <0.70 are poor (17).
For the data analysis, an Excel spreadsheet was used along with GraphPad Prism 6.0 and the statistics package SPSS 21.0 for Mac (SPSS, Inc., Chicago, IL).
Results
ANOVA of means repeated 2×6 times
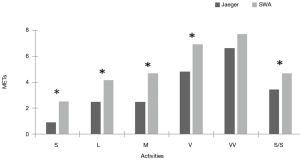
Figure 1 shows the mean MET values obtained for each standardized PA for each monitoring device.
Bland-Altman analysis
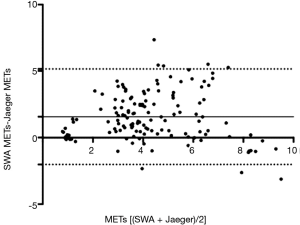
The analysis proposed by Bland-Altman provided BIAS values of 1.56 METs (±1.83 METs) and LOA values from −2.03 to 5.16 METs. Figure 2 shows the results obtained from this analysis.
Heteroscedasticity
This analysis showed a significant association (R2 =0.03; P<0.05), therefore indicating heteroscedasticity.
ROC curves
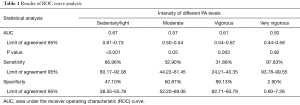
Table 1 shows the data obtained from the ROC curve analysis for each type of PA.
Full table
Conclusions
This is the first study to evaluate the validity and precision of the SWA for estimating EE and PA levels in healthy adults aged 40 to 55 years old. The results show that the SWA yielded significantly different results for all the different activities except for running at 9 km·h-1 (>9 METs). The Bland-Altman analysis yielded a positive BIAS value (1.56), indicating that the SWA overestimates EE compared with the indirect calorimeter for each of the activities. In addition, the LOAs are very wide (7 METs), indicating little concordance between the two methods of estimation. Heteroscedasticity analysis revealed a positive correlation (R2 =0.03; P<0.05), showing that an increase in the magnitude of the variable measured (METs) leads to an increase in the differences of the estimations obtained using the two methods. ROC curve analysis showed that the SWA is not sensitive in estimating the level of PA at high intensities.
Our results are in agreement with those obtained by Fruin and Rankin in 2004, who estimated EE in 20 healthy adults (50% women; 18–35 years of age) while walking on a treadmill at three different intensity levels (80.50 m·min-1, 0° incline; 107.30 m·min-1, 0° incline; 107.30 m·min-1, 5° incline), concluding that the SWA overestimated EE at an incline of zero and underestimated it when the incline was increased (14). In contrast, Johannsen et al. [2007] studied the precision and validity of two monitoring models, the SWA and the SenseWear Mini Armband, compared with the doubly labeled water method for determining EE during daily activities. A total of 30 healthy adults, aged between 24 and 60 years, participated in this study and wore both monitors for a period of 14 days, even when sleeping. The final results showed the precision of the two monitors in measuring EE under these laboratory conditions; however, they concluded that the accuracy needed to be improved for high-intensity activities (18). Similarly, Drenowatz et al. [2011] evaluated the precision of the SWA compared with an indirect calorimeter in estimating EE when performing vigorous and very vigorous PA. A total of 20 young adults (24.3±2.8 years) participated in the study. They ran on a treadmill for 10 minutes at 65.75% and 85% of their VO2 max and for 30 min in the open air at the rhythm they found most comfortable. It was concluded that the limit up to which precise results were obtained was an intensity of approximately 10 METs (19). For a population group of elderly individuals (average age of 82 years), the results demonstrated the validity of the SWA in estimating daily EE (11). Therefore, there is great heterogeneity in the scientific evidence available for the validity and precision of the SWA in EE estimation. This may be a result of the specific characteristics of each population, the monitor firmware, the activities or experimental protocols in each study, or the statistical analyses used in each study.
In another study, the EE of 21 healthy adults was estimated while running on a treadmill for 10 minutes at different intensities, with the aim of comparing the validity of the SWA and other monitors (CSA, TriTrac-R3D, RT3, and BioTrainer-Pro) with an indirect calorimeter. It was concluded that the SWA was the most precise of the monitors for estimating total EE (20). In addition, comparing the results of the present study with those of the study by Santos-Lozano et al. (6), in which the subjects, the experimental protocol, and the statistical analysis were all similar and evaluated the precision of PA level estimation with the Actigraph GT3X accelerometer, it can be seen that the AUC values were similar. The sensitivities of the GT3X and the SWA are very similar, with that of the SWA being slightly better, particularly at light and moderate levels of PA. The specificity of the SWA was better, demonstrating its higher capacity for differentiating false-positives. Therefore, the SWA could provide similar EE and PA level estimation results to those of several accelerometers used both clinically and in research.
It is important to note that one of the limitations of this study is that it was carried out in a laboratory; therefore, the results cannot be extrapolated to EE estimations and PA level classification in normal daily life. However, having controlled each of the standardized PAs, the protocol can be reproduced under similar conditions.
To sum up, the SWA overestimates EE compared with the indirect calorimeter in a population of adults aged between 40 and 55 years. There are also indications that it is insufficiently precise for determining EE, compared with the indirect calorimeter, at high intensities. However, it could provide valid estimations of PA levels at low intensities.
Acknowledgements
We would like to thank the collaboration of the subjects who participated voluntarily in the study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the University’s Human Ethics Committee (University of Zaragoza), and written informed consent was obtained from all patients.
References
- Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334-59. [Crossref] [PubMed]
- Fiuza-Luces C, Garatachea N, Berger NA, et al. Exercise is the real polypill. Physiology (Bethesda) 2013;28:330-58. [Crossref] [PubMed]
- Márquez-Rosa S, Rodríguez Ordax J, Abajo Olea S.. Sedentarismo y salud: efectos beneficiosos de la actividad física. Apunts 2006;83:12-24.
- Vanhees L, Lefevre J, Philippaerts R, et al. How to assess physical activity? How to assess physical fitness? Eur J Cardiovasc Prev Rehabil 2005;12:102-14. [Crossref] [PubMed]
- Aparicio-Ugarriza R, Mielgo-Ayuso J, Benito PJ, et al. Physical activity assessment in the general population; instrumental methods and new technologies. Nutr Hosp 2015;31 Suppl 3:219-26. [PubMed]
- Santos-Lozano A, Santín-Medeiros F, Cardon G, et al. Actigraph GT3X: validation and determination of physical activity intensity cut points. Int J Sports Med 2013;34:975-82. [Crossref] [PubMed]
- Santos-lozano A, Marin PJ, Garatachea N. Accelerometers: Types and applications. In: André PS, Varum H. editors. Accelerometers: Principles, Structures and Applications. New York: Nova Science Publishers, 2013.
- Chen KY, Bassett DR Jr. The technology of accelerometry-based activity monitors: current and future. Med Sci Sports Exerc 2005;37:S490-500. [Crossref] [PubMed]
- Manns PJ, Haennel RG. SenseWear Armband and Stroke: Validity of Energy Expenditure and Step Count Measurement during Walking. Stroke Res Treat 2012;2012:247165.
- Santos-Lozano A, Garatachea N. Tendencias actuales de la acelerometría para la cuantificación de la actividad física. Rev Ib CC Act Fis Dep 2012;1:24-32.
- Mackey DC, Manini TM, Schoeller DA, et al. Validation of an armband to measure daily energy expenditure in older adults. J Gerontol A Biol Sci Med Sci 2011;66:1108-13. [Crossref] [PubMed]
- Almeida GJ, Wasko MC, Jeong K, et al. Physical activity measured by the SenseWear Armband in women with rheumatoid arthritis. Phys Ther 2011;91:1367-76. [Crossref] [PubMed]
- Jakicic JM, Marcus M, Gallagher KI, et al. Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Med Sci Sports Exerc 2004;36:897-904. [Crossref] [PubMed]
- Fruin ML, Rankin JW. Validity of a multi-sensor armband in estimating rest and exercise energy expenditure. Med Sci Sports Exerc 2004;36:1063-9. [Crossref] [PubMed]
- Lamarra N, Whipp BJ, Ward SA, et al. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol (1985) 1987;62:2003-12. [PubMed]
- CareFusion. Jaeger Oxycon Pro manual. Hoechberg, Germany: CareFusion 2009.
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993;39:561-77. [PubMed]
- Johannsen DL, Calabro MA, Stewart J, et al. Accuracy of armband monitors for measuring daily energy expenditure in healthy adults. Med Sci Sports Exerc 2010;42:2134-40. [Crossref] [PubMed]
- Drenowatz C, Eisenmann JC. Validation of the SenseWear Armband at high intensity exercise. Eur J Appl Physiol 2011;111:883-7. [Crossref] [PubMed]
- King GA, Torres N, Potter C, et al. Comparison of activity monitors to estimate energy cost of treadmill exercise. Med Sci Sports Exerc 2004;36:1244-51. [Crossref] [PubMed]


