The subtypes of microscopic colitis from a pathologist’s perspective: past, present and future
Introduction
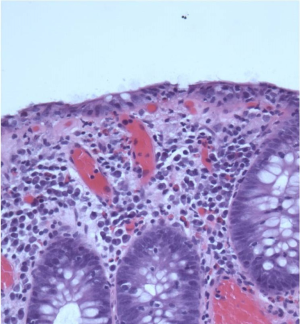
Microscopic colitis (MC) is a chronic inflammatory bowel disease including the morphological subtypes collagenous colitis (CC) (Figure 1) and lymphocytic colitis (LC) (Figure 2). MC is diagnosed as a triad of chronic not bloody diarrhea, a normal/near normal endoscopy and distinctive tissue morphology (1). As the clinical features of CC and LC are indistinguishable (2,3), the pathologists’ contribution to the diagnosis of MC is crucial. The key histological features of CC and LC are shown in Table 1 (4,5).

Full table
Since the establishment of the diagnostic criteria decades ago, changes have occurred in pathologists’ diagnostic approach. Two major changes are described in the following sections: the emergence of a third subgroup of MC and access to additional stains in diagnostics of MC. The changes have crystallized into the challenge as to which criteria and diagnostic methods pathologists should apply. This is followed by a description of Danish pathologists approach to diagnostics of MC and proposals to minimize observer variation and increase diagnostic agreement among pathologists.
Emergence of a third subtype of MC: microscopic colitis incomplete (MCi)
Over the last years, publications have proposed an additional subtype of MC, MCi, comprising incomplete collagenous colitis (CCi) and incomplete lymphocytic colitis (LCi) (6-9). MCi embraces patients with clinical features of MC from whom biopsies fall short of fulfilling the specific histological criteria of CC and LC. Fraser (7) suggested the term “MC not otherwise specified” for this group of patients. The histological key features of MC and MCi are shown in Table 2.

Full table
As patients with MCi seem to benefit from medical treatment with a clinical response equal to patients with MC, it has been suggested to include MCi as a subgroup of MC (2,6). If, however, MCi is accepted as an additional subgroup, the “diagnostic bar” may be set too low and there may be a risk of over diagnosis. On the other hand, if MCi is not accepted and MC is restricted to CC and LC, the “diagnostic bar” may be set too high, as patients with a treatable cause of diarrhea may be missed (10). The concept of MCi raises several questions, the most important being whether the clinicians and pathologists know MCi? And if so, do they also accept it as a diagnostic entity? How do pathologists properly distinguish MC (CC/LC) from MCi (CCi/LCi) and how do they distinguish MCi from the morphology of slight, inflammatory changes?
The emergence of additional stains in the diagnostics of MC
The histopathological diagnose of CC and LC was originally based almost exclusively on heamatoxylin-eosin (HE) stained sections (11,12). While access to and the number of specific stains was scarce, they are now part of routine diagnostic methods in most pathology departments—at least in Denmark. Recent guidelines (1,5) recommend that the histological diagnosis is based on HE stained sections and only in case of diagnostic uncertainty may special stains be applied, which means immunohistochemical stain (CD3) to highlight T-lymphocytes in LC and LCi and a connective tissue stain to highlight the subepithelial collagen band in CC and CCi.
The recommendation of applying special stains “in case of diagnostic uncertainty” raises questions such as: how often are gastrointestinal (GI) pathologists in doubt of the diagnosis? How often do they apply special stains? In any case, as special stains are applied, an unknown proportion of the CC and LC diagnoses are based on stains, which are different from those used when the histological criteria of MC were defined.
The pathologists’ diagnostic dilemma
The emergence of MCi and easy access to special stains has brought about a diagnostic dilemma for the pathologists: to accept MCi and to apply special stains in MC diagnostics or not? That is the question. The diagnostic dilemma is shown diagrammatically in Figure 3.
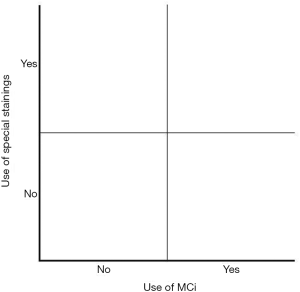
We know little of pathologists’ diagnostic approach to MC and about their position with regard to acceptance of MC. We may however assume that if MCi is accepted and if special stains are often applied, the pathologists are located in the upper right quadrant of Figure 3 and a high incidence of MC/MCi would be expected. If on the other hand, if MCi is not accepted and if special stains are infrequently used, pathologists are located in the lower left quadrant and a low incidence of MC/MCi would be expected.
Knowledge of MCi and use of special stains among Danish Pathologists
This diagnostic dilemma prompted us to prepare a survey among pathologists in Denmark, with the purpose of investigating to which extent MCi is accepted and how a morphological suspicion of MC and MCi is approached diagnostically. A questionnaire was e-mailed to all 15 pathology departments in Denmark, addressed to the consultants responsible for the GI-specimens. All 15 departments participated in the questionnaire, with a response rate of 100%, and 48 GI-pathologists contributed to the survey (13). The survey showed that in terms of:
- HE stained colorectal biopsies with suspicion of CC: 67% of the respondents apply a non-immunohistochemical connective tissue staining, and 30% apply immunohistochemical staining (tenascin) in addition to HE-staining.
- HE stained colorectal biopsies with suspicion of LC: 85% of the respondents use immunohistochemistry (IHC) staining for T-lymphocytes (CD3) in addition to HE staining and 15% do not apply additional stainings at all.
- Awareness of the diagnostic entity of MCi: 50% of the respondents report a consistent use of the term “microscopic colitis obs pro” indicating knowledge and acceptance of MCi.
A morphological suspicion of MC thus conspicuously increases the use of special stains/immunohistochemical stains among pathologists in Denmark. Together with the high level of acceptance of MCi, the Danish pathologists are located in the upper right quadrant of Figure 3 and this may be an explanation of the high incidence of CC and LC in Denmark (3,14).
Considering the recommendation to apply special stains “in case of diagnostic uncertainty” the widespread use of particularly CD3 in Denmark (85%) is noteworthy (13). Rather than indicating a fundamental diagnostic uncertainty among pathologists in Denmark, it is an expression of the common diagnostic approach: if special stains are available at the pathology departments, the stains are naturally applied to optimize and specify the histological diagnose.
Pathologists’ observer variation in the diagnostics of MC and MCi
Pathologists’ observer variation in diagnostic routine including MC is well known (15). Regarding histopathological entities, it is important that the histological criteria are well defined, reproducible and accepted by the pathologists. We performed a study to assess pathologists’ ability to distinguish MC, MCi and normal biopsies (16). The material included slides of biopsies from 125 subjects, covering the diagnoses of: (I) CC; (II) LC; (III) CCi/LCi (MCi); (IV) IBD or infectious inflammatory changes; and (V) normal colon mucosa. Based on a single HE-stained slide, the blinded material was independently reviewed by three pathologists without knowledge of the clinical diagnosis. Four months later the material was relabeled, blinded and reinterpreted. The study showed that intra- and interobserver agreement is high for discriminating cases of MC and MCi (CC, LC, CCi/LCi) from cases of non-MC (IBD/ infectious inflammation and normal), while the ability to discriminate MCi (CCi and LCi) from MC (CC and LC) was lower.
Efforts to minimize observer variation in diagnostics of LC and LCi: CD3 staining
The survey among Danish pathologists revealed that approximately 50% of the GI-pathologists accept the concept of MCi and they often apply additional stains in MC diagnostics. The latter may be caused by the acceptance of MCi, giving rise to more cases of diagnostic uncertainty. The survey also indicated that when only HE-stained slides are available, discriminating CCi/LCi from CC/LC is difficult. It brings one to ask the question: how is it possible to reduce the number of cases of diagnostic uncertainty as well as interobserver variation?
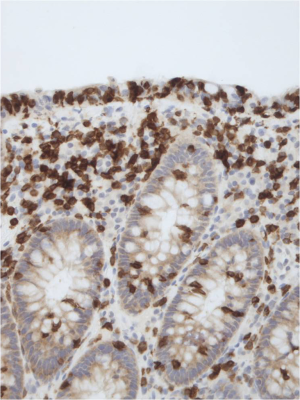
It is commonly accepted that the true number of intraepithelial lymphocytes (IELs) of colon biopsies are better visualized by a CD3 immunohistochemical staining. For comparison, the HE-stained biopsy of Figure 2 is stained with CD3 in Figure 4. It is obvious that many T-lymphocytes, not visible in the HE-stained slide, show up in the CD3 stained slide.
We therefore conducted a study to evaluate whether a supplementary CD3 staining may contribute to a higher diagnostic agreement among pathologists, thus providing a rationale for recommending CD3 staining (17). Four pathologists evaluated 156 archived biopsies, originally diagnosed as LC or LCi. Each pathologist assigned one of three diagnoses (LC, LCi, or IBD/infectious inflammation) to all cases at two independent assessments. At the first assessment, only HE stainings were available, at the second assessment a supplementary CD3 staining was also available.
Our study indicates that in cases of LC and LCi a supplementary CD3 staining (I) increases the diagnostic agreement between pathologists; (II) changes the diagnosis in a proportion of cases based on HE stained sections; (III) reduces the number of cases primarily considered as LCi. Adding a CD3 stain reveals that many of the LCi cases do fulfill the morphological LC criteria. The latter is in accordance with a decline in number of patients diagnosed in as LCi after the introduction of CD3 staining in routine clinical practice in the Region of Zealand in Denmark (Munch LK, personal communication). Furthermore, a diagnosis of LC based on HE staining was only rarely changed by a supplementary CD3. It is therefore recommended to add a CD3 stain in cases of diagnostic uncertainty and always before signing out a specimen as LCi.
Although additional CD3 stains makes it easier to recognize the true number of lymphocytes, the diagnostic disagreement among pathologists is not completely eliminated. We therefore decided to explore automated image analysis of tissue sections as a tool to minimize pathologists’ interobserver variation.
Efforts to minimize observer variation in diagnostics of MC and MCi: automated image analysis
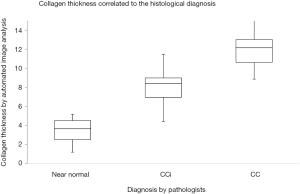
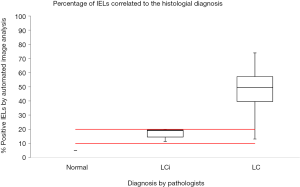
Automated image analysis is useful in research of diseases characterized by detection of accumulation of specific cell types, for instance T-lymphocytes’ in lung allograft (18) or in assessment of the collagen fibers organization in cutaneous scar tissue (19). To explore the utility of automated image analysis in diagnostics of MC and MCi, software was developed to measure the subepithelial collagen band and to assess the number of IELs of colon biopsies (20,21). Two training sets of colon biopsies: (I) 10 biopsies covering CC/CCi/normal; and (II) 10 biopsies LC/LCi/normal were used to develop software to assess the thickness of the collagenous band and the number of IELs. The two study sets consisted of (I) 75 biopsies with CC, CCi and normal (25 each) stained with a connective tissue staining (Van Gieson stain) and (II) 59 biopsies with LC and LCi (38 and 21 respectively) stained with CD3. Four pathologists individually reassessed the biopsies and categorized them into one of the above-mentioned categories and the results were compared with measurements made by automated image analysis. The study showed that measuring the thickness of the collagenous band and assessing the number of IELs by automated image analysis is consistent and congruent with the diagnosis made by trained GI-pathologists (Figures 5,6). In the LC/LCi study two cases of normal colon biopsies were accidently included in the study set. Both cases were diagnosed as normal by the pathologists as well as by automated image analysis, thus emphasizing automated image analysis to be a reliable tool to assess the number of IELs in colon biopsies. Accordingly, automated image analysis may be applied in difficult diagnostic cases, e.g., differentiating MC (CC/LC) from MCi (CCi/LCi) or differentiating MCi from biopsies with slight inflammatory changes or from normal biopsies. Being an objective and reliable diagnostic tool, automated image analysis may also contribute to achieve uniform histopathological diagnosis in multicenter studies—provided the stains used by the contributors are identical.
Achieving greater knowledge of MC: the European PRO-MC collaboration
The exact disease course of MC is largely unknown and prospective studies are needed. To adequately assess the disease course, a large, complete and non-selected patient cohort is required. To obtain this an international collaboration to study microscopic colitis has been established: the European prospective registry for Microscopic Colitis in Europe (the European PRO-MC collaboration) (22). The purpose of the PRO-MC collaboration is a systematic and prospective accumulation of individual, long-term disease courses of MC. The PRO-MC registry is web-based, aiming to generate insight to the long-term disease course of MC, to identify predictive markers of disease course and treatment outcome. The European Microscopic Colitis group (EMCG) (23) with support of the United European Gastroenterology (UEG) LINK award has initiated the PRO-MC collaboration (24).
A slide kit describing the morphological and diagnostic criteria of MC and MCi has been developed in collaboration between EMCG and the European Society of Pathology in order to increase the awareness of MC and to improve data validity. Before participating in the database, the centers are stimulated to distribute the slide kit among their pathologists. The slide kit as well as the review article on histopathological aspects of MC (5) represents the pathologists’ educational material of the PRO-MC collaboration.
Information on biopsy location, degree of lamina propria inflammation and number of IELs, thickness of collagenous band, the types of stains used and the conclusive histological diagnosis are registered in the pathology form of the database. These histopathological data will enable comparison of the pathology departments’ diagnostic approach to MC and inform about their position in the diagram of Figure 3. However, a prerequisite to compare data is that pathologists agree on the diagnostic approach. As mentioned above, in Denmark MCi is accepted by 50% of GI-pathologists and there is widespread use of special stains which may contribute to the high incidence of MC in Denmark, while other countries do not accept the diagnosis of MCi.
Summary on the diagnostics of MC and MCi—past, present and future
In this article, the changes in diagnostic criteria and diagnostic methods of MC have been described. The number of subgroups has changed from two to three (Table 2). Previously one single stain, the HE-stain, was applied to the sections. Recent guidelines recommend HE-stained sections, but in case of doubt pathologists may apply additional stains. Regarding the survey presented above, it is our view that pathologists interpret the recent guidelines as follows: “In full blown cases of LC and CC diagnosis can be made easily on HE-stained sections, but in cases of diagnostic doubt additional stainings are recommended.” In this way, most Danish pathologists are located in the upper right quadrant of the diagram of Figure 3. This diagnostic approach to MC is irreversible, meaning that if pathologists first have become used to apply special stains routinely, it is unrealistic to turn back time applying HE stain as the only staining method.
Data from the ongoing PRO-MC collaboration will inform on the participating MC centers acceptance of MCi and use of special stains, and subsequently their location in the diagram of Figure 3. Follow-up on clinical data of patients of the four quadrants will show whether patients of the upper quadrant are over diagnosed or if patients of the lower quadrant are under diagnosed and which of the four quadrants are closest to “the true diagnostic method”.
The PRO-MC collaboration has the potential to serve as a basis for several future research topics.
A recent article highlights the importance of the lamina propria inflammation (3), as biopsies obtained prior to the MC diagnosis often reveal an increased level of lympho-plasmacellular infiltration. In addition, the effect on budesonide on diarrhea (1,2) indicates that inflammation of the lamina propria which is shared by all subtypes of MC is essential to the disease course. Today automatic image analysis is a valuable research tool, being an objective and reproducible method to eliminate inter-observer variability which is shown also in our studies (20,21). This encourages to use image analysis to study the increased lympho-plasmacellular infiltration of the lamina propria, quantifying the number and composition of the various cell types.
It is generally accepted, that pathologists are the gateway to an accurate diagnosis of diseases—MC being a valuable example. However, a prerequisite to get valid clinical data in diseases where pathologists take part in diagnostics is that they agree on the histopathological diagnostic criteria and staining methods. Future studies, national as well as international, including those within the framework of PRO-MC collaboration will contribute to achieve a common diagnostic approach and to learn more about the disease course of MC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Münch A, Aust D, Bohr J, et al. Microscopic colitis: Current status, present and future challenges: statements of the European Microscopic Colitis Group. J Crohns Colitis 2012;6:932-45. [Crossref] [PubMed]
- Bjørnbak C, Engel PJ, Nielsen PL, et al. Microscopic colitis: clinical findings, topography and persistence of histopathological subgroups. Aliment Pharmacol Ther 2011;34:1225-34. [Crossref] [PubMed]
- Rasmussen J, Engel PJ, Wildt S, et al. The Temporal Evolution of Histological Abnormalities in Microscopic Colitis. J Crohns Colitis 2016;10:262-8. [Crossref] [PubMed]
- Aust DE. Microscopic colitis: histological criteria. In: Miehlke S, Münch A, eds. Microscopic colitis—creating awareness for an underestimated disease. Basel: Falk Workshop, 2012:23-6.
- Langner C, Aust D, Ensari A, et al. Histology of microscopic colitis-review with a practical approach for pathologists. Histopathology 2015;66:613-26. [Crossref] [PubMed]
- Munck LK. Incomplete microscopic colitis: a broader and clinically relevant perspective. In: Miehlke S, Münch A, eds. Microscopic colitis—creating awareness for an underestimated disease. Basel: Falk Workshop, 2012:27-31.
- Fraser AG, Warren BF, Chandrapala R, et al. Microscopic colitis: a clinical and pathological review. Scand J Gastroenterol 2002;37:1241-5. [Crossref] [PubMed]
- Fernández-Bañares F, Casalots J, Salas A, et al. Paucicellular lymphocytic colitis: is it a minor form of lymphocytic colitis? A clinical pathological and immunological study. Am J Gastroenterol 2009;104:1189-98. [Crossref] [PubMed]
- Goldstein NS, Bhanot P. Paucicellular and asymptomatic lymphocytic colitis: expanding the clinicopathologic spectrum of lymphocytic colitis. Am J Clin Pathol 2004;122:405-11. [Crossref] [PubMed]
- Langner C. Colorectal normal histology and histopathologic findings in patients with chronic diarrhea. Gastroenterol Clin North Am 2012;41:561-80. [Crossref] [PubMed]
- Lindström CG. 'Collagenous colitis' with watery diarrhoea--a new entity? Pathol Eur 1976;11:87-9. [PubMed]
- Lazenby AJ, Yardley JH, Giardiello FM, et al. Lymphocytic ("microscopic") colitis: a comparative histopathologic study with particular reference to collagenous colitis. Hum Pathol 1989;20:18-28. [Crossref] [PubMed]
- Engel PJ, Holck S, Engel U. A questionnaire on the diagnosis of microscopic colitis in Denmark. J Crohns Colitis 2016;10:S227-8.
- Bonderup OK, Wigh T, Nielsen GL, et al. The epidemiology of microscopic colitis: a 10-year pathology-based nationwide Danish cohort study. Scand J Gastroenterol 2015;50:393-8. [Crossref] [PubMed]
- Limsui D, Pardi DS, Smyrk TC, et al. Observer variability in the histologic diagnosis of microscopic colitis. Inflamm Bowel Dis 2009;15:35-8. [Crossref] [PubMed]
- Fiehn AM, Bjørnbak C, Warnecke M, et al. Observer variability in the histopathologic diagnosis of microscopic colitis and subgroups. Hum Pathol 2013;44:2461-6. [Crossref] [PubMed]
- Fiehn AM, Engel U, Holck S, et al. CD3 immunohistochemical staining in diagnosis of lymphocytic colitis. Hum Pathol 2016;48:25-31. [Crossref] [PubMed]
- Krustrup D, Iversen M, Martinussen T, et al. Time elapsed after transplantation influences the relationship between the number of regulatory T cells in lung allograft biopsies and subsequent acute rejection episodes. Transpl Immunol 2014;31:42-7. [Crossref] [PubMed]
- Quinn KP, Golberg A, Broelsch GF, et al. An automated image processing method to quantify collagen fibre organization within cutaneous scar tissue. Exp Dermatol 2015;24:78-80. [Crossref] [PubMed]
- Fiehn AM, Kristensson M, Engel U, et al. Automated image analysis in the study of collagenous colitis. Clin Exp Gastroenterol 2016;9:89-95. [Crossref] [PubMed]
- Engel PJ, Fiehn AK, Kristensson M, et al. Automated Image Analysis of Lymphocytes in Lymphocytic Colitis. J Clin Exp Pathol 2015;5:261.
- Munch A, Verhaegh B. Cross-border scientific projects run by UEG national member societies reduce health inequalities across Europe. United European Gastroenterol J 2016;4:478. [Crossref] [PubMed]
- Available online: http://www.emcg-ibd.eu/index.html
- Available online: http://www.emcg-ibd.eu/european-registry-promc.html