ALK on my mind: alectinib takes an early lead in managing intracranial disease in non-small cell lung cancer with ALK rearrangements
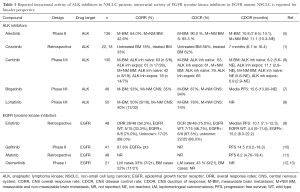
Non-small cell lung cancers (NSCLC) harboring oncogenic anaplastic lymphoma kinase fusions (ALK+) embody the paradigm and success of precision medicine. Despite high overall response rates (ORR) with the first ALK inhibitor crizotinib, a pattern of central nervous system (CNS) failure emerged, highlighting the need for CNS-specific study and assessment. In fact, the CNS is the first site of progressive disease (PD) in nearly 70% of ALK+ patients taking crizotinib (1). Unlike crizotinib and ceritinib, alectinib is not a substrate of P-glycoprotein, a key efflux transporter that hinders drug penetration through the blood-brain barrier (BBB) and may partly underlie observations of pharmacologic failure (2,3). The ratio of alectinib to plasma in cerebrospinal fluid (CSF) approaches 0.75 indicating a very high degree of CNS penetration (4). Early small data sets showed that the intracranial response rate of alectinib ranged from 40% to 57% (Table 1) (14). Additionally, alectinib was reported to have activity in ALK+ NSCLC patients with leptomeningeal disease (15,16).
Full table
In the October 2016 issue of the Journal of Clinical Oncology, Gadgeel et al. significantly expanded on the understanding of CNS response to alectinib in a pooled analysis from two single-arm phase II studies (NP28761 and NP28673) in patients with ALK+ NSCLC who were previously treated with crizotinib (5). Both studies evaluated the objective response rate of alectinib 600 mg twice daily by mouth in ALK+ NSCLC patients with prior crizotinib treatment. Secondary end points included CNS overall response rate (CORR), CNS disease control rate (CDCR), and CNS duration of response (CDOR) (Table 1).
The analysis consisted of 136 patients with baseline CNS metastases (60% of the overall study populations) who were assessed for intracranial response. Fifty patients (37%) had measurable CNS disease at baseline. Ninety-five patients (70%) had prior CNS radiotherapy (55 patients had CNS radiotherapy more than 6 months prior to alectinib initiation). Median follow-up time was about 1 year. CNS response and progression were assessed per RECIST version 1.1 by independent review committee consisting of neuroradiologists who were blinded to systemic response. The proportion of patients undergoing MRI, CT or both MRI and CT were 62.5%, 27.9% and 9.6%, respectively. Brain scans were taken every 6 weeks in the NP28761 study and every 8 weeks in NP28673. For patients with baseline measurable CNS disease, CORR was 64.0% (95% CI, 49.2–77.1%), CDCR was 90.0% (95% CI, 78.2–96.7%), with a median CDOR of 10.8 months (95% CI, 7.6 to 14.1 months). For patients with measurable and/or non-measurable baseline CNS disease, CORR was 42.6% (95% CI, 34.2–51.4%), CDCR was 85.3% (95% CI, 78.2–90.8%), and median CDOR was 11.1 months (95% CI, 10.3 months to not evaluable) (Table 1). When stratified by prior radiotherapy (pre-specified) responses were seen in 35.8% (95% CI, 26.2–46.3%) with prior radiotherapy (n=95) and 58.5% (95% CI, 42.1–73.7%) individuals without prior radiotherapy (n=41). Complete intracranial responses were observed in 18% of patients with and 49% of patients without prior radiotherapy. Similar to prior studies, alectinib was well tolerated with 5.9% patients discontinuing from the study due to intolerable adverse events (5).
This pooled analysis represents the largest dataset examining the CNS activity of alectinib and is strengthened by both its prospective collection and independent radiology committee assessment. Both studies employed the same alectinib dosing schedule and had similar protocols with respect to imaging frequencies for response assessment. The authors should be commended for focusing on a significant knowledge gap in ALK+ NSCLC, the optimal management of CNS metastases. Overall, their results confirmed previous observations that alectinib has robust intracranial activity, which is irrespective of radiation history and comparable to systemic response. Compared with the first-generation ALK inhibitor crizotinib, which achieves CNS control in 56% of ALK inhibitor naïve patients, alectinib has favorable toxicity and efficacy profiles though head to head CNS activity first line comparisons are lacking. The additional newer generation ALK TKIs have demonstrated favorable CNS activity though large analyses are still ongoing (7,8). Relevant to the therapeutic sequencing of ALK inhibitors is the time to CNS progression, something not captured in earlier trials. The ongoing first line alectinib trials may confirm that alectinib prevents or delays the development of CNS metastases to a greater degree than crizotinib (17).
As mentioned by the authors, potential weaknesses of their analysis included small sample size for some subgroups and the single arm design of the two studies. The study does not provide tumor mutational profiles or CSF concentration and future clinical trials would benefit from detailed CNS pharmacokinetic (i.e., drug absorption, CFS drug level) and pharmacodynamics studies to refine the causes of CNS progression. Emerging data show that various EML4-ALK fusion variants may predict differential response and disease control to crizotinib (18). For instance, patients with EML4-ALK variant 1 had similar ORR to crizotinib (74% vs. 63%) but higher disease control rate (DCR) (95% vs. 63%) and longer median progression free survival (PFS) (11.0 vs. 4.2 months) than individuals with other variants (18). Whether specific fusion partners and/or breakpoint variant biology hold up in CNS-specific analyses or investigating if alectinib can overcome the biologic variation remains to be determined.
Dosing strategies to overcome poor CNS activity have met some success in EGFR mutant NSCLC, and were not formally examined in the analysis by Gadgeel and colleagues (19). While alectinib 600 mg twice daily by mouth yields respectable intracranial response and tolerability it is unclear if higher or “pulse” dose would achieve superior response rate. Recently, Gainor et al. reported that alectinib dose escalation (900 mg twice daily by mouth) re-induced CNS tumor response in two patients with ALK+ NSCLC who experienced CNS relapse on standard dose alectinib (600 mg twice daily by mouth) (20). The results from the frontline J-ALEX and ALEX alectinib trials (vs. crizotinib) will further clarify the intracranial activity and may inform differential CNS response/control by dosing as 300 mg BID is used in J-ALEX and 600 mg BID in the ALEX trial (NCT02075840). The intracranial efficacy of alectinib also raises arguments for using alectinib monotherapy in well-selected ALK+ NSCLC patients with BM over standard therapies (i.e., whole brain radiotherapy, stereotactic body radiation therapy or surgical resection). While de-intensifying brain radiation-based therapies in oncogene-driven NSCLC is attractive it is not yet supported by prospective studies. In an analogous situation in EGFR mutant NSCLC erlotinib alone in TKI naïve patient with CNS mets resulted in inferior OS (25 months) and intracranial PFS (17 months) when compared incorporation of radiotherapy (21). Subgroup analysis demonstrated that patients who received upfront SRS followed by erlotinib had the longest median OS (46 months), followed by the upfront WBRT group (30 months) (21). Meta analyses in EGFR mutant disease has suggested that cranial RT followed by erlotinib may be superior to upfront erlotinib in patient with CNS mets (22). Although biologically different, similar studies in ALK+ NSCLC will be important to support the observation that TKI can be used alone for CNS metastasis in selected patients.
Overall Gadgeel et al. provide convincing evidence for alectinib in ALK+ patients with brain metastases and early subgroup analyses from the frontline J-ALEX trial vs. crizotinib suggest clear superiority. We expect alectinib to take a lead in the management of ALK+ NSCLC, particularly in the presence of CNS metastases. Ongoing trials with second and third generation inhibitors and the optimal role of radiation will further refine the management of CNS disease.
Acknowledgments
None.
Footnote
Conflicts of Interest: SJK has received honoraria from Foundation Medicine, Inc. and Eli Lilly. PNT has no conflicts of interest to declare.
References
- Costa DB, Shaw AT, Ou S-HI, et al. Clinical experience with crizotinib in patients with advanced ALK-rearranged non–small-cell lung cancer and brain metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. [Crossref] [PubMed]
- Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014;74:1023-8. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Gadgeel SM, Shaw AT, Govindan R, et al. Pooled Analysis of CNS Response to Alectinib in Two Studies of Pretreated Patients With ALK-Positive Non–Small-Cell Lung Cancer. J Clin Oncol 2016;34:4079-85. [Crossref] [PubMed]
- Kim D-W, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol 2016;17:1683-96. [Crossref] [PubMed]
- Solomon B, Bauer T, Felip E, et al. Safety and efficacy of lorlatinib (PF-06463922) from the dose-escalation component of a study in patients with advanced ALK+ or ROS1+ non-small cell lung cancer (NSCLC). J Clin Oncol 2016;34:9009.
- Wu Y-L, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG–0803). Ann Oncol 2013;24:993-9. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Schuler M, Wu YL, Hirsh V, et al. First-Line afatinib versus chemotherapy in patients with non–small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol 2016;11:380-90. [Crossref] [PubMed]
- Yang J. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): updated results from BLOOM, a Phase I study. Abstract 9002. In annual meeting of the American Society of Clinical Oncology 2016;3-7.
- Ahn M, Kim D, Kim T, et al. Phase I study of AZD3759, a CNS penetrable EGFR inhibitor, for the treatment of non-small-cell lung cancer (NSCLC) with brain metastasis (BM) and leptomeningeal metastasis (LM). In ASCO Meeting Abstracts 2016;9003.
- Tran PN, Klempner SJ. Focus on Alectinib and Competitor Compounds for Second-Line Therapy in ALK-Rearranged NSCLC. Front Med (Lausanne) 2016;3:65. [Crossref] [PubMed]
- Ou S-HI, Sommers KR, Azada MC, et al. Alectinib induces a durable (> 15 months) complete response in an ALK-positive non-small cell lung cancer patient who progressed on crizotinib with diffuse leptomeningeal carcinomatosis. Oncologist 2015;20:224-6. [Crossref] [PubMed]
- Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol 2015;10:232-6. [Crossref] [PubMed]
- Nokihara H, Hida T, Kondo M, et al. Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): Primary results from the J-ALEX study. In ASCO Annual Meeting Proceedings 2016;9008.
- Yoshida T, Oya Y, Tanaka K, et al. Differential crizotinib response duration among ALK fusion variants in ALK-positive non–small-cell lung cancer. J Clin Oncol 2016;34:3383-9. [PubMed]
- Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364-9. [Crossref] [PubMed]
- Gainor JF, Chi AS, Logan J, et al. Alectinib Dose Escalation Reinduces Central Nervous System Responses in Patients with Anaplastic Lymphoma Kinase–Positive Non–Small Cell Lung Cancer Relapsing on Standard Dose Alectinib. J Thorac Oncol 2016;11:256-60. [Crossref] [PubMed]
- Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naive Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 2017;35:1070-7. [PubMed]
- Soon YY, Leong CN, Koh WY, et al. EGFR tyrosine kinase inhibitors versus cranial radiation therapy for EGFR mutant non-small cell lung cancer with brain metastases: a systematic review and meta-analysis. Radiother Oncol 2015;114:167-72. [Crossref] [PubMed]


