New endoscopic approach of anti-fibrotic therapy for inflammatory bowel disease
Introduction
Inflammatory bowel disease (IBD), comprised of Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic, progressive, destructive disease of the gastrointestinal tract characterized by the presence of extensive ulceration and mucosal inflammation in the gut. Despite a great progress in modern anti-inflammatory therapies including immuno-suppressants and biologics, intestinal fibrosis is a frequent complication in the natural history of IBD, as up to one-third of CD patients and about 5% of UC patients develop strictures in the clinical course of the disease (1-12). In CD, chronic inflammation induces transmural damage which causes accumulation of extracellular matrix (ECM) and expansion of mesenchymal cells, finally leading to intestinal strictures. In UC, increased amount of collagen and thickening of the muscularis mucosa have been identified in the colon, with consequent shortening and increased rigidity of the colon. This implies that control of intestinal inflammation alone does not necessarily affect the associated fibrotic process. There are no standard anti-fibrotic medical treatments for IBD, and dilation of intestinal strictures with endoscopy or surgical treatment plays an important role in managing the strictures of IBD (3-10). Therefore, treatment goals of IBD include not only symptom control alone but prevention of intestinal fibrosis with structural bowel damage, that is, bowel tissue remodeling (11,12).
In response to inflammation-driven bowel damage, “fibrotic healing” occurs instead of adequate mucosal repair, leading to co-existence of mucosal defect like ulcer and fibrosis at local sites. In clinical practice, refractory ulcers often associated with fibrosis not only in drug-resistant peptic ulcer and esophageal ulcer post submucosal dissection but also in IBD (3,4,13,14). Co-existence of ulcer and fibrosis is thus characterized by “tissue remodeling” in the gut and is one of the causes of difficulty to treat local lesions in IBD. Since multifactorial process is involved in tissue remodeling, therapeutic approaches against single mediator alone would not be sufficient to normalize fibrotic lesions (3,4,14,15). Integrated anti-fibrotic/tissue remodeling strategy that simultaneously targets inflammatory mediators, profibrotic factors and tissue intrinsic changes is recommended as the most successful way to treat this highly complex and difficult-to-manage pathology (15). We therefore focused on ECM molecules that modulate the function of multiple mediators at sites of injury and hypothesized that blockade of certain ECM molecule could normalize the fibrotic architecture en bloc.
Carbohydrate sulfotransferase 15 (CHST15) and chondroitin sulfate-E (CS-E) in tissue remodeling
Mechanism of CHST15/CS-E-mediated fibrosis
CHST15, formerly known as N-acetylgalactosamine 4-sulfate 6-O-sulfotransferase (GalNAc4S-6ST), is a type II transmembrane Golgi protein that biosynthesizes highly sulfated disaccharide units (E-units) of chondroitin sulfate (CS), which binds to various functional proteins and pathogenic microorganisms (16-18). CHST15/CS-E axis is reported be be involved in fibrosis through two major mechanisms; activation of fibroblasts and formation of collagen fibrils (Figure 1). CS-E acts as the co-receptor for Wnt for signal transduction of Wnt/Frizzled pathway of fibroblasts. CS-E acts as the direct receptor for receptor for advanced glycation end product (RAGE) on fibroblasts, suggesting that CS-E directly stimulates RAGE-mediated fibrotic pathway (16). CS-E is also reported to bind CD44, chemokines like MCP-1/CCL2 and SDF-1/CXCL12 and growth factors like PDGF and TGF-β indicating the involvement in adhesion, migration and proliferation of fibroblasts (17). In addition, CS-E was shown to enhance fibril formations of collagen as well as Aβ(19,20). At the same time, CS proteoglycan was shown to inhibit proteolytic degradation of collagen and Aβ(19,20). CS-E binds type V collagen, which is increased in submucosa of CD patients (21-24), suggesting that matrix CS-E strengthens CS-E-collagen interaction thereby solid fibrotic formation which is an obstacle of tissue repair.
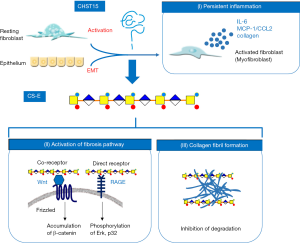
Mode of action of CHST15 inhibitor STNM01 in fibrosis
STNM01 is a synthesized double stranded RNA oligonucleotide that selectively inhibits the expression of CHST15 gene (25,26). STNM01 (or experimental grade of CHST15 siRNA with the same sequence; hereafter we refer both as “STNM01”) suppressed CHST15 mRNA in human colon and lung fibroblast cell lines in vitro and reduced the expression of CHST15 mRNA in the colon of mouse DSS colitis (25), the esophagus of pig esophageal stricture post ESD (27), the lung of mouse pulmonary fibrosis (28) and the heart of rat myocarditis (29). CHST15 protein was also reduced by STNM01 in in vivo disease models. STNM01 reduced the amount of CS/sulfated Glycosaminoglycan (GAG) in the human colon fibroblast and in in vivo disease models. The amount of E-unit of CS/sulfated GAG chain was selectively reduced in the human colon fibroblast (25).
STNM01 reduced the mRNA expressions of vimentin, α-SMA and Wnt3 by TGF-β-stimulated human fibroblast cell line as well as human colon cancer cell line, indicating that STNM01 inhibits the activation of fibroblasts from both resident fibroblasts and epithelial cells through epithelial mesenchymal transition (EMT) (25). STNM01 enhanced the mRNA expressions of E-cadherin and TGF-β induced EMT-counteracting molecule BMP-7, suggesting EMT-inhibiting effect (25). Reduced production of IL-6, MCP-1/CCL2 and collagen was observed in fibroblast cell lines by the treatment with STNM01. STNM01 also reduced the accumulation of fibroblast as well as the expression of α-SMA in in vivo disease models, supporting its inhibitory effect on fibroblasts. Interestingly, STNM01 reduced the expression of Loxl2, an enzyme that catalyzes the collagen cross-linking, suggesting and involvement in regulating fibrinolytic pathway (28).
Since STNM01 possess dual targets, CHST15 and its specific product CS-E, the mode of action in IBD is summarized in Figure 1. In response to tissue injury, CHST15 is induced by activated fibroblasts from resident/recruited fibroblasts or through EMT. Activated fibroblasts/myofibroblasts produce IL-6, MCP-1, collagen and CS-E into ECM. Matrix CS-E directly binds to RAGE or acts as a co-receptor for Wnt/Frizzled on fibroblasts, then further stimulates fibrosis pathway in an autocrine or paracrine manner. Matrix CS-E directly promotes collagen fibrils and also inhibits degradation of established fibrils, leading to the inhibition of fibrinolytic property. STNM01 therefore could shut down the vicious cycle of fibrogenesis. In addition, as excessive fibrils would be an obstacle to epithelial healing, restoration or promotion of fibrinolytic activity is prerequisite for epithelial regeneration. STNM01 would enhance the degradation of established collagen fibrils, and this also contribute to treat tissue remodeling.
A novel pancolonic delivery as a translatable application of oligonucleotide
Endoscopic submucosal injection
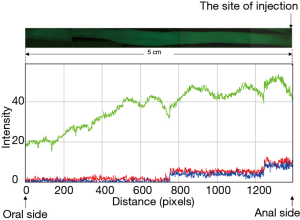
To translate therapeutic potential of oligonucleotide into clinical practice, it is important to increase targeting and retention of siRNA at sites of injury in patients. We have established a novel pancolonic delivery in mice by an approach utilizing submucosal injection through endoscopy (25). Only one site-injection enables siRNA to rapidly spread in a circumferential manner and at the same time, to diffuse from rectum to cecum in mice (25). Imaging analysis of the intensity of fluorescence signal in stereoscopic microscopy was performed after one-site injection of green fluorescence-labeled siRNA in normal mouse colon (Figure 2). The average intensity, which was indicated as the intensity of Green channel, at 5 cm (oral side) away from the site of injection (anal side) was approximately 40% of that at the site of injection (Figure 2). This suggested that the amount of siRNA at 5 cm away from the site of injection was also approximately 40% at the injection site. Thus, if the injection is performed at 10 cm-interval, the theoretical amount of siRNA at the middle position (5 cm from the injection sites) will be 80% in total. We also demonstrated previously that injected siRNA could retain within matrix space and to be incorporated into cells in a sustained manner (25). Our discovery indicates that the direct injection of siRNA into ECM without facing bloodstream is the most important key technique to achieve successful retention of siRNA and continuous supply to cells from matrix fiber.
Clinical application for IBD and evidence of target CHST15 reduction in patients
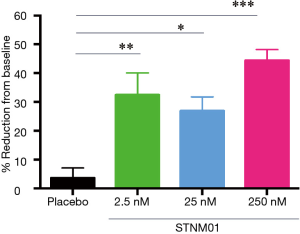
Given the novel pancolonic siRNA delivery, we next tried to translate it to clinical study for IBD. For CD, we took an approach to focal injection as most CD patients have localized ulcers and lesions (26). In accordance with “lifting-up” technique before endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) against localized gastrointestinal caners, STNM01 was submucosally administered to surround the periphery of the target, localized lesion (Figure 3) in a phase 1 first-in-patient clinical trial (26). There were 8 total injection sites. The volume of injection was 1 mL/site per injection, thus 8 mL/one lesion in total (Figure 3). The effect of the target CHST15 reduction was also evaluated by immunological staining of biopsy specimens from the edge of the target ulcer. The grade of CHST15 staining was scored (26) and the % reduction of CHST15-positive score was evaluated at day 30 from the baseline (Figure 4). Compared to the placebo group who did not show the reduction of CHST15, the STNM01-treatment groups showed the significant reductions at day 30 after single injection of STNM01 (Figure 4). Of these, the highest dose of STNM01 (250 nM) showed the superior silencing efficiency (Figure 4), indicating that CHST15 immunostaining is a good method to identify the drug efficiency in patients. There was no drug-related side effect as well as inflammation at the injection sites, indicating that the method is safe as expected.
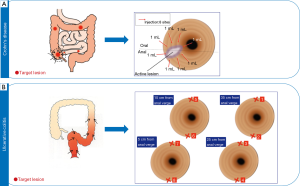
Distinct from CD, UC depicts a continuous lesion from rectum. We have therefore conducted distinct injection model for UC in a phase 2a study. STNM01 is evenly administered in a 10 cm-interval (Figure 3) based on the pancolonic delivery observed in mice (Figure 2). There were total 8 site injection sites. Two site-injections (upper and bottom sites) at 35, 25, 15 and 5 cm from anal verge for left-sided colitis were performed. The volume of injection was 2 mL/site per injection, thus 16 mL/left-side colon in total (Figure 3). Theoretically, at least 40 cm length of colon can be fully covered by this approach. The results will be presented soon.
Endoscopic MH and histological fibrosis as efficacy measures
Induction of quick MH response by STNM01 with anti-fibrotic action
Phase 1 first-in-patient trial was conducted for patients with CD who do not respond to conventional treatment including biologics (hereafter, “non-healer”) and endoscopy as well as histology were investigated as secondary endpoints (26). Figure 5 shows a typical patient depicting deep ulcer despite intensive treatment with infliximab for 2 years. Biopsies were performed from the edge of ulceration or the center of scar during the study period. Masson trichrome staining revealed that the existence of severe fibrosis, inflammation and crypt destruction at baseline, indicating infliximab refractory (Figure 5A). Surprisingly, only 7 days after single injection with STNM01, the refractory ulcer reduced dramatically and red regenerative mucosa appeared surrounding healing ulcer (Figure 5, lower left panel). The biopsy forceps are used for estimating the size of ulcer and clearly demonstrated that the size of ulcer exhibited over 50% reduction in diameter 7 days after injection. Histology of biopsy specimens demonstrated that the repression of fibrosis, reduction of inflammatory infiltrate and re-appearance of goblet cells with fine crypt architecture, supporting the endoscopic findings of the regenerative mucosa (Figure 5B).
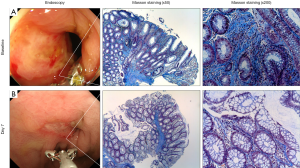
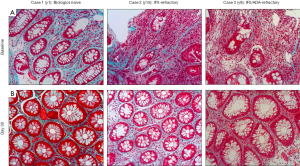
Figure 6 showed histology of 3 patients who received STNM01 at an another clinical site. Case 1 was an early CD patient as the disease duration was 1 year (y1; Figure 6, left panels). The patient received 5-ASA and corticosteroid but depicted drug-resistant ulcer, then was enrolled in the study before using biologics [biologics naive]. The histology showed that inflammation and crypt destruction were relatively weak but fibrosis was evident, indicating that fibrotic reaction already occurred in such an early disease phase and was uncontrollable by 5-ASA and corticosteroid alone. At day 30, however, the histology was almost normalized with repression of fibrosis by STNM01. Case 2 was infliximab-refractory patient with disease duration of 15 years (y15; Figure 6, middle panels). Diffuse inflammation within fibrotic space and crypt destruction were evident at baseline, supporting infliximab-refractory. At day 30, the patient showed reduced fibrosis/inflammation and improved crypt architecture including re-appearance of goblet cells. Case 3 was infliximab/adalimumab-double failure with disease duration of 8 years (y8; Figure 6, right panels). At baseline, heavily inflammation with more fibrotic space and severer crypt destruction were observed. At day 30, fibrosis was clearly repressed and crypt was also repaired although minor focal inflammation still existed. All patients here also showed clear endoscopic MH, suggesting that repression of histological fibrosis was predicted by endoscopic MH.
Mechanism why anti-fibrotic drug STNM01 induces MH in refractory CD patients
Tissue remodeling consists of (I) failure to eliminate the inciting factors, (II) obstacle to mucosal repair and (III) persistent activation of inflammatory cells and fibroblasts (15). Collagen fibril in fibrotic tissue is an obstacle to epithelial regeneration/MH. As prerequisite for epithelial regeneration, the blockade of CS-E by STNM01 could enhance the degradation of established collagen fibril and restore fibrinolytic activity. In addition, STNM01 inhibits further fibrosis pathways, thus could shutdown malignant cycle of fibrogenesis. Whether STNM01 directly acts on the epithelial cells to promote their proliferation or not was not established yet. But STNM01 increases BMP-7 which counteracts to TGF-β-mediated EMT. This also contributes to enhancing MH activity. Although STNM01 showed reduced inflammatory infiltrates in patients and in animal models, it is also still unclear whether anti-inflammatory lead-out by STNM01 was secondary to anti-fibrotic action or due to other distinct mechanism of action. The effects of STNM01 on epithelium and inflammation should be investigated further in order to clarify the mechanism of its integrated anti-tissue remodeling activity.
One question arose whether repression of fibrosis may increase the risk of perforation. In animal models, STNM01 did not affect normal fibroblasts and tissue architecture. Major activity of STNM01 is on activated fibroblasts but not on normal fibroblasts that do not show higher level of CHST15. Important information was achieved from pig model of esophageal stricture after ESD which is characterized by both fibrosis and atrophic change of muscularis proper (27). STNM01 showed no muscle layer defect, supporting the low risk of perforation or fistula. Interestingly, STNM01 possess rather protective effect to muscle layer. Indeed, there was no sign of perforation and fistula in phase 1 study and its long-term observation over 1 year (26). Thus, anatomy-based, appropriate anti-fibrosis therapy does not increase the risk of perforation or fistula because it does not impact on muscle layer.
Future perspective
Fibrosis in IBD is a largely unresolved clinical problem, but no anti-fibrotic therapies are available at present. Multiple obstacles have hampered the progress of developing novel anti-fibrotic drugs in IBD. To overcome these obstacles, we have conducted a series of studies. To summarize, our research road map is shown in Figure 7. We have identified a new target CHST15 as a key pathogenic factor that has a potential to regulate multi-factors in tissue remodeling. To increase the specificity, we selected siRNA as a feasible compound and took suitable application for siRNA delivery. This is a new endoscopic submucosal injection possessing pancolonic delivery. We also established animal model that can bring insights into the pathogenesis of intestinal fibrosis (30) and demonstrated the therapeutic effect of STNM01 in view of anti-fibrosis as well as MH (25). As the balance between MH and fibrosis would contribute to disease outcome in tissue remodeling in IBD, we have investigated both endoscopic MH and histological fibrosis in animal and patients and shown endoscopy would be a feasible tool to predict the anti-fibrotic efficacy. We thus consider both endoscopic finding of MH and expression of CHST15 in the local site of intestine as a golden marker which predict fibrosis and response to therapy.
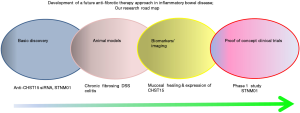
Based on the repression of pre-established fibrosis in patients with CD by the treatment with STNM01, CHST15 is demonstrated as a good therapeutic target for intestinal fibrosis in human. Considering anti-fibrotic actions of CHST inhibition in other organs like heart and lung (27,28), further modification to systemically applicable formulation including oral or systemic siRNA or screening of small molecule inhibitors is of great interest in the future.
Conclusions
Our studies document the translational practice from nonclinical studies into first-in-patient study by establishing the specific therapeutic target that could modulate multiple factors in tissue remodeling, clinically feasible application for siRNA and tissue-specific endpoints. The patient results also demonstrate an opportunity for new endoscopic anti-fibrosis therapy in IBD.
Acknowledgements
The authors are grateful to Professors Hitoshi Asakura (Niigata University), Toshifumi Hibi (Kitasato University), Kazuichi Okazaki (Kansai Medical University), Yasuo Suzuki (Toho University), Markus F. Neurath (Erlangen University) and Raja Atreya (Erlangen University) for critical medical advises during the translational practice, and professors Kazuyuki Sugahara (Hokkaido University) and Kenichi Watanabe (Niigata University of Pharmacy) for their professional nonclinical studies. We also thank to Dr. Yukinori Sameshima (Sameshima Hospital) and Dr. Tsuneo Fukushima (Matsushima hospital) for their performance in Phase 1 study.
Footnote
Conflicts of Interest: K Suzuki is an inventor of a patent related to submucosal injection. H Yoneyama founded Stelic Institute & Co., Inc. in 2004. Stelic filed and issued patents related to the treatment of intestinal fibrosis/inflammatory bowel disease with oligonucleotide-based medicine targeting CHST15.
References
- Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007;369:1641-57. [Crossref] [PubMed]
- Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut 2012;61:1619-35. [Crossref] [PubMed]
- Rieder F, Fiocchi C. Intestinal fibrosis in IBD--a dynamic, multifactorial process. Nat Rev Gastroenterol Hepatol 2009;6:228-35. [Crossref] [PubMed]
- Friedman SL, Sheppard D, Duffield JS, et al. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med 2013;5:167sr1. [Crossref] [PubMed]
- de Bruyn JR, Meijer SL, Wildenberg ME, et al. Development of Fibrosis in Acute and Longstanding Ulcerative Colitis. J Crohns Colitis 2015;9:966-72. [Crossref] [PubMed]
- Rieder F, de Bruyn JR, Pham BT, et al. Results of the 4th scientific workshop of the ECCO (Group II): markers of intestinal fibrosis in inflammatory bowel disease. J Crohns Colitis 2014;8:1166-78. [Crossref] [PubMed]
- Burke JP, Mulsow JJ, O'Keane C, et al. Fibrogenesis in Crohn's disease. Am J Gastroenterol 2007;102:439-48. [Crossref] [PubMed]
- Gordon IO, Agrawal N, Goldblum JR, et al. Fibrosis in ulcerative colitis: mechanisms, features, and consequences of a neglected problem. Inflamm Bowel Dis 2014;20:2198-206. [Crossref] [PubMed]
- Rieder F, Zimmermann EM, Remzi FH, et al. Crohn's disease complicated by strictures: a systematic review. Gut 2013;62:1072-84. [Crossref] [PubMed]
- Rieder F. Toward an antifibrotic therapy for inflammatory bowel disease. United European Gastroenterol J 2016;4:493-5. [Crossref] [PubMed]
- Iacucci M, Ghosh S. Looking beyond symptom relief: evolution of mucosal healing in inflammatory bowel disease. Therap Adv Gastroenterol 2011;4:129-43. [Crossref] [PubMed]
- Pineton de Chambrun G, Peyrin-Biroulet L, Lémann M, et al. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol 2010;7:15-29. [Crossref] [PubMed]
- Kakushima N, Fujishiro M, Kodashima S, et al. Histopathologic characteristics of gastric ulcers created by endoscopic submucosal dissection. Endoscopy 2006;38:412-5. [Crossref] [PubMed]
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest 2007;117:524-9. [Crossref] [PubMed]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 2012;18:1028-40. [Crossref] [PubMed]
- Mizumoto S, Yamada S, Sugahara K. Molecular interactions between chondroitin-dermatan sulfate and growth factors/receptors/matrix proteins. Curr Opin Struct Biol 2015;34:35-42. [Crossref] [PubMed]
- Yamada S, Sugahara K. Potential therapeutic application of chondroitin sulfate/dermatan sulfate. Curr Drug Discov Technol 2008;5:289-301. [Crossref] [PubMed]
- Bonnans C, Chou J, Werb Z. Remodelling the extracellular matrix in development and disease. Nat Rev Mol Cell Biol 2014;15:786-801. [Crossref] [PubMed]
- Gupta-Bansal R, Frederickson RC, Brunden KR. Proteoglycan-mediated inhibition of A beta proteolysis. A potential cause of senile plaque accumulation. J Biol Chem 1995;270:18666-71. [Crossref] [PubMed]
- Castillo GM, Lukito W, Wight TN, et al. The sulfate moieties of glycosaminoglycans are critical for the enhancement of beta-amyloid protein fibril formation. J Neurochem 1999;72:1681-7. [Crossref] [PubMed]
- Kvist AJ, Johnson AE, Mörgelin M, et al. Chondroitin sulfate perlecan enhances collagen fibril formation. Implications for perlecan chondrodysplasias. J Biol Chem 2006;281:33127-39. [Crossref] [PubMed]
- Takagaki K, Munakata H, Kakizaki I, et al. Domain structure of chondroitin sulfate E octasaccharides binding to type V collagen. J Biol Chem 2002;277:8882-9. [Crossref] [PubMed]
- Graham MF, Diegelmann RF, Elson CO, et al. Collagen content and types in the intestinal strictures of Crohn's disease. Gastroenterology 1988;94:257-65. [Crossref] [PubMed]
- Belmiro CL, Souza HS, Elia CC, et al. Biochemical and immunohistochemical analysis of glycosaminoglycans in inflamed and non-inflamed intestinal mucosa of patients with Crohn's disease. Int J Colorectal Dis 2005;20:295-304. [Crossref] [PubMed]
- Suzuki K, Arumugam S, Yokoyama J, et al. Pivotal Role of Carbohydrate Sulfotransferase 15 in Fibrosis and Mucosal Healing in Mouse Colitis. PLoS One 2016;11:e0158967. [Crossref] [PubMed]
- Suzuki K, Yokoyama J, Kawauchi Y, et al. Phase 1 Clinical Study of siRNA Targeting Carbohydrate Sulphotransferase 15 in Crohn’s Disease Patients with Active Mucosal Lesions. J Crohns Colitis 2017;11:221-8. [Crossref] [PubMed]
- Sato H, Sagara S, Nakajima S, et al. Prevention of esophageal stricture after endoscopic submucosal dissection, using siRNA-based silencing of carbohydrate sulfotransferase 15 in pig. Endoscopy. [Epub ahead of print].
- Kai Y, Tomoda K, Yoneyama H, et al. Silencing of carbohydrate sulfotransferase 15 hinders murine pulmonary fibrosis development. Mol Therapy Nucleic Acid in press.
- Watanabe K, Arumugam S, Sreedhar R, et al. Small interfering RNA therapy against carbohydrate sulfotransferase 15 inhibits cardiac remodeling in rats with dilated cardiomyopathy. Cell Signal 2015;27:1517-24. [Crossref] [PubMed]
- Suzuki K, Sun X, Nagata M, et al. Analysis of intestinal fibrosis in chronic colitis in mice induced by dextran sulfate sodium. Pathol Int 2011;61:228-38. [Crossref] [PubMed]


