Category 1 external quality assessment program for serum creatinine
IntroductionOther Section
In recent years, the need to standardize analytical methods has significantly increased, because patients might be treated by physicians receiving results from multiple laboratories within the healthcare system. This fact implies the need to assure interchangeability of patients` results through improving standardization of laboratory tests. Standardization has a special relevance for serum creatinine, since its measurement is essential in the estimation of the renal function and may have a clear impact on patient care (classification and treatment of patients with renal chronic disease) (1).
In 1998, the European directive 98/79/CE (2) gave to the external quality assessment programs (EQAP) the responsibility to monitor the performance of in vitro diagnosis medical devices within the European market.
In 2011, EQAP were classified into six categories (3), according to their ability to verify the degree of standardization of the participating measurement procedures. A category 1 EQAP uses commutable control materials with target values assigned by certified reference methods, being a valuable tool to evaluate the degree of standardization and to estimate the accuracy of the participating laboratories (4).
The EQAP classification of the Spanish Society of Clinical Biochemistry and Molecular Pathology (SEQC) belongs to fifth category (replicate analyses of non-commutable materials with no values assigned by reference system), which allows us to evaluate the imprecision and to make a comparison with other laboratories with the same analytical method.
In 2015, the Spanish Society of Laboratory Medicine (SEQC) in collaboration with the Dutch Foundation for the Stichting Kwaliteitsbewaking Medische Laboratorium Diagnostiek (SKLM) carried out a pilot category 1 EQAP for 17 biochemistry analytes (alanine aminotransferase, aspartate aminotransferase, bilirubin, calcium, chloride, creatinine kinase, creatinine, alkaline phosphatase, gamma glutamyltransferase, glucose, lactate dehydrogenase and glomerular filtration rate (eGFR, obtained from the equation used for each laboratory), magnesium, potassium, total protein, sodium urate). This study focuses only on creatinine results
Aim
To evaluate the standardization degree for serum creatinine testing in Spanish laboratories
MethodsOther Section
A set of six control materials, at different concentrations, prepared by SKML were distributed in a single express shipment at −80 °C and delivered within a 24 hours’ time period to 87 laboratories all over Spain. They were fresh-frozen human serum samples (commutable) with values assigned by certified reference methods covering a wide measurable range for the most of the analytes.
The instructions given by SEQC to all participant laboratories were: Samples had to be maintained at −20 °C for all over the study period (maximum two weeks). They were measured in duplicate for 6 consecutive days for each material. Every laboratory sends 12 results to SEQC office using its current online system, to be individually and collectively evaluated.
Creatinine target values were measured by isotope dilution mass spectrometry (IDMS) by the Deutsche Vereinte Gesellschaft für Klinische Chemie und Laboratoriumsmedizin e.V. (DGKL) laboratory (Bonn, Germany) which is included in JCTLM database.
For each laboratory and control sample, percentage deviations of the mean of paired values versus the true value (PD) were calculated, as well as coefficients of variation (CV) calculated from duplicated test samples. PD and CV obtained were compared with the quality specifications for total allowable error (TEa) derived from biological variation, at desirable level (5). These are widely used criteria, defined in the Stockholm consensus conference (6) and confirmed in the Milan strategic conference (7).
When results are grouped (i.e., according to method), the percentage deviation of the median of each group versus the reference value is calculated for each control sample. Then, results are compared against the specification derived from biological variation for systematic error (5).
ResultsOther Section
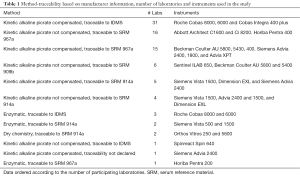
A total of 1,044 creatinine results were obtained in this pilot study. The number of laboratories and instruments participating in the study, classified according to a method-traceability code system, are presented in Table 1. From the total, two laboratories did not correctly describe their methodology (one user of Siemens Advia 2400 and one user of Roche Cobas 8000 did not know their method or their traceability, as can be seen in Table 1). Two types of reports were performed:
Full table
Individual laboratory reports
Results were published in the same website as the remaining SEQC-EQAP (http://contcal.org). This site is restricted to participants and guarantees confidentiality of data by using lab-code identification.
For each control sample and individual result, the percentage deviation (PD) versus the true value is reported. Every PD higher than the desired limit for total error derived from biological variation is depicted in red colour, whereas those that fell within this limit are depicted in green.
Intra-laboratory imprecision, expressed in terms of coefficient of variation (CV) is calculated from duplicated samples and averaging the six concentration levels (lots).
The results obtained by the group of laboratories using same method and traceability are also shown in the individual laboratory report. For each control sample, group-CV and group-PD are shown.
Overall report
After removing methods with only one participant as well as the two laboratories without declared traceability, participants are divided into 12 different types of method-traceability combination. The most frequent method used was kinetic alkaline picrate compensated, traceable to IDMS followed by not compensated, traceable to serum reference material (SRM) 967a (Table 1).
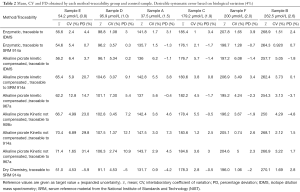
Table 2 shows the statistics (mean, CV and PD) obtained for each control sample and by each method-traceability combination.
Full table
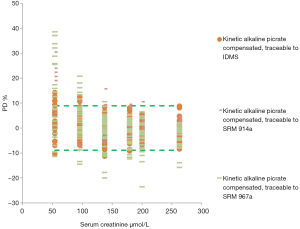
Figure 1 depicts the mean of percentage deviation for each method-traceability group compared with the reference value, for all samples distributed. This represents a general view of the global systematic error for the entire measured concentration interval Desirable limit for the systematic error derived from biological variation (4%), is depicted in dotted green lines.
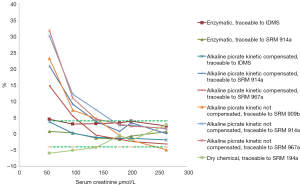
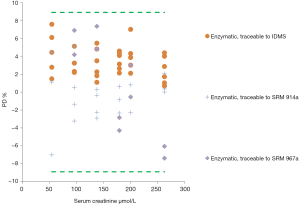
If we analyse method by method separately, we can conclude that only enzymatic methods got all the individual results of each laboratory within the acceptable limits of desirable TAe Figure 2.
Although estimated PD average of the compensated alkaline picrate method traceable to IDMS fell into the desirable limits, not all individual results fulfil the goal for TAe (Figure 3). Moreover, compensated alkaline picrate methods with different traceability than IDMS, showed more dispersion of data and more results were outside the limits.
Related to alkaline picrate non compensated method, all individual results were outside desirable limits at low concentrations (Figure 4).
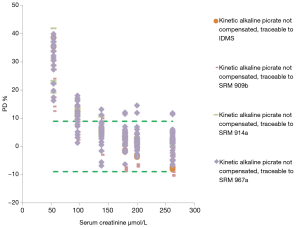
It is necessary to highlight, as a general view, that alkaline picrate kinetic method (compensated or non compensated) showed a remarkable overestimation of creatinine from 10% to 35% at creatinine concentration below 100 µmol/L, where clinical decision making are frequently made especially in women and pediatric population. These results are independent of the analytical platform used for creatinine testing.
DiscussionOther Section
The main conclusion arisen from this study is that only enzymatic methods seem to obtain suitable results, regardless of their traceability and of the instrument applied related to the intended clinical use of creatinine in clinical practice. This had already been noticed by Weykamp et al. (8), who observed a strong positive bias for creatinine determined by the alkaline picrate (Jaffé) method, probably caused by endogenous interfering components; so they recommended not using the Jaffé method. Also, Jassam et al. (9), running another EQAP in 87 European laboratories, confirmed that only the enzymatic method reached the specifications recommended by the National Kidney Disease Program Education (KDIGO) (10).
The wide variability in the traceability chain used by in vitro diagnostic devices seems not to have a great influence in standardization of creatinine testing. What is important is to provide clear information regarding traceability and not only to show the analytical principle. Laboratories have found important troubles when codifying their methods traceability. In order to avoid misinterpretations, we have recoded them upon information provided by manufacturers. However, a big effort should be made to clarify this information in order to facilitate laboratory knowledge of their methods.
Furthermore, the healthcare impact of false positive creatinine results is of utmost importance because of the consequent false low glomerular filtration rate, which could generate wrong clinical decisions. Subsequently, unnecessary complementary diagnostic tools would be requested to the patients with an added needless economical expense as well as potential damage to the patient. This situation is even more important in women and pediatric population healthcare.
Further outcomes studies should be done to assess the impact of inaccurate creatinine results over patient misclassification and then directly over the clinical decision making. In our country a big effort should be made to promote laboratories to change their procedures and to use enzymatic creatinine methods, in order to achieve a satisfactory standardization degree for this relevant analyte.
In conclusion, to participate in a category 1 EQAP is a valuable tool to assess the standardization of laboratory tests. The lack of standardization for creatinine, evidenced in our country, could potentially lead to errors in interpreting laboratory reports; this situation should be radically changed.
AcknowledgementsOther Section
None.
FootnoteOther Section
Conflicts of Interest: The authors have no conflicts of interest to declare.
ReferencesOther Section
- Myers GL, Miller WG, Coresh J, et al. Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem 2006;52:5-18. [Crossref] [PubMed]
- Directive 98/79/EC of the European Parliament and of the Council of 27 October 1998 on in vitro diagnostic medical devices. OJ L 331 of 7 December 1998.
- Miller WG, Jones GR, Horowitz GL, et al. Proficiency testing/external quality assessment: current challenges and future directions. Clin Chem 2011;57:1670-80. [Crossref] [PubMed]
- Jansen R, Jassam N, Thomas A, et al. A category 1 EQA scheme for comparison of laboratory performance and method performance: An international pilot study in the framework of the Calibration 2000 project. Clin Chim Acta 2014;432:90-8. [Crossref] [PubMed]
- Minchinela J, Ricós C, Perich C, et al. Biological variation database, and quality specifications for imprecision, bias and total error (desirable and minimum). The 2014 update. Biologic Variation Database 2014. Available online: https://www.westgard.com/biodatabase-2014-update.htm
- Petersen PH, Fraser CG. Strategies to set global analytical quality specifications in laboratory medicine: 10 years on from the Stockholm consensus conference. Accreditation and Quality Assurance 2010;15:323-30. [Crossref]
- Sandberg S, Fraser CG, Horvath AR, et al. Defining analytical performance specifications: Consensus Statement from the 1st Strategic Conference of the European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem Lab Med 2015;53:833-5. [Crossref] [PubMed]
- Weykamp C, Secchiero S, Plebani M, et al. Analytical performance of 17 general chemistry analytes across countries and across manufacturers in the INPUtS project of EQA organizers in Italy, the Netherlands, Portugal, United Kingdom and Spain. Clin Chem Lab Med 2017;55:203-11. [Crossref] [PubMed]
- Jassam N, Weykamp C, Thomas A, et al. Post-standardization of routine creatinine assays: are they suitable for clinical applications. Ann Clin Biochem 2016. [Epub ahead of print]. [Crossref] [PubMed]
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements 2013;3.


