Environmental asbestos exposure and risk of mesothelioma
Introduction
Malignant mesothelioma is an aggressive form of cancer that typically originates in the pleural but can also occur in the peritoneum, pericardium and around the testes. Asbestos exposure is the only established risk factor known to be causally related to mesothelioma. Mesothelioma and other asbestos related diseases are commonly attributed to occupational exposures. Even in countries that no longer extract asbestos and where commercial uses have declined, past occupational exposures remain the predominant driver for mesothelioma mortality. However, in recent decades, numerous studies have focused on environmental (i.e., non-occupational) asbestos exposure pathways and risk of mesothelioma. While occupational exposures are on the wane in some countries, it can be argued that these environmental sources of exposure will account for larger proportions of mesothelioma incidence. Thus, it is imperative to characterize these increasingly important and emerging environmental exposure sources with the likelihood that these pathways will be a growing feature of future mesothelioma risk.
Asbestos is a fibrous mineral with physical and chemical properties that make it resistant to heat and degradation. Such properties have resulted in asbestos fibers being used for a variety of industrial applications, and commercial asbestos is still extracted from open pits. Indeed, world production is expected to remain steady in the near future at approximately two million metric tons with the predominant sources being Brazil, China, Kazakhstan and Russia (1). Regulated asbestos falls under two classifications: the amphibole group that includes anthophyllite, actinolite, tremolite, amosite and crocidolite and the serpentine group that includes chrysotile. These classifications serve a useful purpose for characterizing extraction products, fiber types used in industry, and, to some degree, differences in disease risk by fiber type. However, as discussed in the section on naturally occurring asbestos (NOA), reliance on such classifications is limiting when considering environmental exposures and risk of mesothelioma (2). Thus, the term asbestos will be used in this review as the more general term that includes asbestos fibers and the non-regulated fibers that have physical properties similar to commercial asbestos.
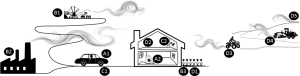
In this review we will consider four main categories of non-occupational asbestos exposure (see Figure 1). We begin with exposure pathways that are most closely connected to occupational exposures and industrial practices. Specifically, we consider take-home exposures from asbestos workers, or para-occupational exposure, followed by environmental exposures to communities with large asbestos-related industrial operations. We then discuss the potential for environmental exposure to asbestos containing products, focusing particularly on fixed-in-place products found in residential settings and vulnerable to disturbance. Finally, we explore the past decades of studies in communities, typically small, that have experienced extensive NOA exposures and opportunities for translating that knowledge to more recently discovered NOA near large communities. Each of these exposure categories will be presented within the context of what we currently understand with respect to the associated risk for mesothelioma among exposed populations.
Para-occupational exposure
The term para-occupational exposure refers to an asbestos-exposed worker serving as a vector for the transport of fibers to the household setting. Other terms used in this context include household contact, take-home exposure or domestic exposure. The latter term can be confused with the residential exposure pathways discussed in the subsequent section that includes asbestos-contaminated insulation or soils. Para-occupational exposure is more specific to the indirect asbestos exposure from a high-risk occupational setting to the household or other setting where people, typically family members, interact with the worker. Thus, among the four classes of environmental exposure considered in this review, para-occupational fiber exposure has the closest connection to the occupational setting.
A few different routes have been suggested for para-occupational exposure, but data on related exposures concentrations are limited. The most common activity attributed to para-occupational exposure is laundering of the contaminated clothes from workers. Some simulation studies measured air concentrations during handling of asbestos-contaminated clothing and suggest that potential take-home exposures to household contacts are a fraction, perhaps 1%, of occupational exposures (3). However, studies are not available to quantify airborne exposure concentrations for laundry handling where the worker contact was in a high exposure occupation [for review see (4)]. Notably, lung tissue asbestos burden among para-occupationally exposed women with mesothelioma was in a similar range to the fiber burden among mesothelioma cases among men with moderate occupational exposure such as construction (5). Although laundering is often the focus of para-occupational exposures, other activities such as cleaning will disturb dust containing asbestos that was transported by the worker to the home. This route of exposure is poorly understood and will vary greatly according to the occupational source. The worker’s vehicle also serves as a route of para-occupational to household members and others. Potential exposures to household contacts from contaminated household dust or vehicles can be integrated into exposure assessments if individual-level data are collected on contact frequency with these routes (6).
Over the past few decades across many countries several hundred mesothelioma cases have been reported among family members of workers in industries with likely asbestos exposure. Associated industries included mining, shipbuilding, asbestos cement manufacturing and insulators among others. These reports have been enumerated in a recent review, and over half of the reported cases, predominantly pleural mesothelioma, were confirmed at autopsy (7). Described here are features of some of the case reports with both a large number of mesothelioma cases and a mixture of asbestos-related industries that account for the source of the take-home exposure. An early case series report described ten of 52 female cases of mesothelioma that had husbands or fathers with occupational asbestos exposure (8). All ten cases, two of whom also worked in textiles or shoemaking, reported history of regularly hand-laundering their husband’s or father’s work clothes. A matched analysis with non-cancer controls yielded an odds ratio (OR, and 95% confidence interval (CI)) of 10 (1.4–37) for this para-occupational exposure activity. In northeastern Italy a review of 94 female mesothelioma cases indicated 34 who had cleaned work clothes of family members that were occupationally exposed, primarily in shipyards (9). In the United States from 1990 to 2005, 32 confirmed mesothelioma cases were identified among household contacts, predominantly family members, of asbestos-exposed workers (10). The report excluded any cases that had potential occupational asbestos exposure or residential history near asbestos-related industries. Cases were identified through records of law firms representing claims, thus the number reported by Miller during this period is expected to be an unquantifiable undercount.
Several observational studies have been conducted to investigate para-occupational exposures and risk of mesothelioma. A case control study investigated 185 mesothelioma deaths in Yorkshire, England (11). When limiting cases to those without likely or possible asbestos occupational exposure, para-occupational exposure was observed in 50% of cases (17/34) and 19% of controls (11/58). The risk estimates and precision vary widely depending on the strategy of including or excluding occupations according to likely, possible and unlikely categories. A larger case-control study, including all of Britain, included 622 living mesothelioma patients and 1,420 population-based controls (12). A statistically significant two-fold greater risk was observed for mesothelioma cases with para-occupational asbestos exposure prior to age 30 and no direct occupational exposure compared to controls. Findings were similar for women and men with such exposure histories. In a six-city case-control mesothelioma study an analysis of men and women with no direct occupational asbestos exposure investigators observed an odds ratio (and 95% CI) of 4.8 (1.8–13) for domestic exposure (i.e., combined para-occupational exposure and home characteristics indicating potential residential exposures to asbestos materials) (13). This analysis excluded those with exposures attributed to residential proximity to industrial asbestos facilities. The authors also were able to demonstrate a crudely characterized dose-response effect when categorizing domestic exposure according to levels of probability and estimated levels of intensity. A cohort study of 1,780 women married to employees of an asbestos cement plant detected 11 mesothelioma cases, yielding a standardized incidence ratio (and 95% CI) of 25.19 (12.57–45.07) (14). None of the 11 women had occupational exposure from the asbestos cement plant, but one of the women did work as a goldsmith.
Studies that have focused on a particular community or region with a more specific industrial source for the para-occupational exposure have benefitted from more precise, and at times quantifiable, exposure assessment. A population-based case-control study in Casale Monferrato, a northwest Italy community with occupational and environmental exposures related to an asbestos cement plant, showed an OR (and 95% CI) of 2.2 (1.2–4.0) for mesothelioma and history of living with an occupationally exposed family member (15). More specifically with respect to exposure route, the risk estimate was similar (OR=2.3) when limiting the analysis to family members of cases that were reported to have brought work clothes home for cleaning. The investigators also were able to quantify exposure in this population, representing a substantial improvement over similar studies that have relied primarily on qualitative exposure assessment. For non-occupational exposures, including both proximity of residence to source and domestic/para-occupational pathways, the four exposure categories ranged from <0.1 fiber/mL-years to ≥10 fiber/mL-years. The analysis showed a monotonic dose-response relationship and OR (and 95% CI) of 23.3 (2.9–187) for the highest category relative to the lowest. When limiting the analysis to only domestic/para-occupational pathways the monotonic dose-response remained evident, but the risk estimates were less precise [OR=6.8 (0.9–52.5 for the highest exposure category)]. The quantified exposure assessment approach was based on expert assessment and subject to misclassification, but such error would likely bias the risk estimates toward the null.
As with the Casale Monferrato studies of para-occupational exposures from a specific asbestos cement plant source, some investigations have assessed mesothelioma associated with take-home exposures from a specific mining operation. The largest such report is from the crocidolite mine in Wittenoom, Western Australia where 30 mesothelioma cases were detected among women living in the township between 1943 and 1992 and not involved in the asbestos mining or milling operations (16). Of these cases, 26 (90%) lived with an asbestos mining or milling worker and 16 (53%) had washed the clothes of an asbestos miner or miller, resulting in elevated but not statistically significant hazard ratios. It is likely that these mesothelioma cases were exposed via a combination of para-occupational exposure, airborne environmental exposure from the mining and milling operations and from exposure to tailings from the mine that were used for paved areas and soils in the town. Mesothelioma incidence was also evaluated in this community (17). These analyses also showed elevated, but not statistically significant, risk estimates for women with mesothelioma who reported washing the clothes of, or living with, an asbestos worker.
A recent meta-analysis, including many of the above-described studies yielded a summary OR (and 95% CI) of 5.0 (2.5–10) for para-occupational exposure and mesothelioma (4). Summary risk estimates were similar when stratifying by study type (i.e., case-control or cohort) and when further stratifying case-control studies based on inclusion or exclusion of subjects that also had potential occupational exposure. Thus, despite the uncertainty with respect to quantifiable exposure levels to household contacts and the difficulty for some study sites in being able to distinguish between para-occupational exposure and neighborhood exposure from an asbestos industry point source (see following section) the evidence that para-occupational exposures are associated with mesothelioma is quite strong.
Environmental exposure from industrial operations
The second area of environmental asbestos exposure is related to para-occupational exposure as it is similarly tied to the extraction, processing or industrial use of asbestos. Numerous residential communities that provide the workers for these industries also can be subject to neighborhood contamination from these commercial enterprises. Indeed, for studies of non-occupational exposure pathways it can be difficult to disentangle para-occupational exposures from residential exposures attributed to industrial point sources. Exposures from these point sources can occur via airborne emissions through loading, processing, ventilation, or waste disposal activities or via the local use of waste products from the facility (e.g., mine tailings) for roads, soil amendments or other purposes. Here we will consider studies at national, regional and local levels that have evaluated mesothelioma risk and residential proximity to industrial asbestos sources. We will also describe two communities with documentation of extensive use of asbestos-contaminated industrial waste products as examples of special cases of neighborhood exposures from local industrial sources.
Few studies at the national scale have evaluated mesothelioma and environmental exposures from asbestos-related industries. As described above, a population-based case-control study in Britain found an increased risk of mesothelioma associated with para-occupational exposure (12). However, this study found no association between mesothelioma and residential proximity to potentially hazardous sites (e.g., asbestos factory or shipyards). A review of mesothelioma standardized incidence ratios (SIRs) by district in France showed the highest SIRs among women with no indication of asbestos exposure occurred in many of the same districts that had high SIRs among women with occupational exposures (18). The authors suggested that these and similar findings from this ecological study support the hypothesis that mesothelioma among women without identified occupational or para-occupational asbestos exposure were attributed to environmental asbestos exposures. In the United States, mesothelioma incidence and mortality was described in 70 communities that had receiving or processing operations for an asbestos-contaminated vermiculite material originating from Libby, MT (19). Elevated standardized mortality ratios (SMRs) were identified in seven of the sites, at the city level, but these elevations were only observed among male cases. Elevated SIRs were observed in seven sites. Only one site had both elevated SMR and elevated SIR, and these analyses were not able to evaluate individual level occupational or environmental exposure.
The Italian national registry of malignant mesothelioma (ReNaM) provides one of the most comprehensive characterizations of individual-level exposure by occupational, para-occupational or environmental pathways. A recent ReNaM evaluation of incident mesothelioma cases detected the largest spatial clusters located in areas with large asbestos cement plants or shipyards (20). Among three communities with large asbestos cement manufacturing facilities, 38% (467/1,217) of cases were women and 20% (198/1,006) of all cases with characterized exposure sources were attributed to environmental exposures. Environmental exposure included both para-occupational exposure and exposure attributed to residential proximity to the asbestos cement plant, so attribution to cases without para-occupational exposures is not clear. Mesothelioma case clusters were also identified in communities with large shipbuilding industry, but small proportions of these clusters were attributed to environmental exposures. Another mesothelioma cluster was identified in Cirie, a small community with asbestos mining operations. A high proportion of these cases were women (38%), and 12% (8/67) were described as environmental cases, again including both para-occupational exposure and proximity to the mine.
Several studies described mesothelioma risk across communities or regions with a variety of asbestos-related industrial sources. A multi-center study of six communities in Italy, Spain and Switzerland was able to separate out confounding from para-occupational exposure (13). After excluding subjects with para-occupational exposure, the investigators found that living within 2000 meters of asbestos mines, asbestos cement plants, asbestos textiles, shipyards, or brakes factories was associated with a high increased risk for mesothelioma (OR (and 95% CI) = 12 (2.8–47)). The authors also were able to demonstrate a dose-response effect when categorizing environmental exposure according to levels of probability and estimated levels of intensity. A study in Yorkshire, England with multiple identified industrial sources of asbestos did not find an increased risk for mesothelioma associated with environmental exposure estimates after eliminating subjects exposed occupationally or para-occupationally (11). Over 200 industrial point sources were identified and residential proximity to any of these were characterized. Although some attempt was made to dichotomize sources according to whether or not the manufacture of asbestos goods was more certain, the study as with many of this type had a high potential for exposure misclassification.
Three communities in northwest Italy, Casale Monferrato, Bari and Broni, have been the subject of extensive study due to the presence of large asbestos cement manufacturing plants and the high occurrence of mesothelioma among residents without direct occupational exposure. As indicated above, these three communities were recognized as spatial mesothelioma clusters with high proportions of female cases and non-occupational exposures (20). Several studies in these communities have demonstrated dose-response associations based on proximity to asbestos cement plants and risk of mesothelioma. In Broni an evaluation of standardized incidence ratios (SIR) for mesothelioma were compared by geography among only those cases without occupational or para-occupational exposure (21). The SIR (and 95% CI) was 13 (9.6–17) in Broni, 6.1 (3.9–9.4) in adjacent communities, and 1.2 (0.4–2.9) in more distant surrounding communities. In Casale Monferrato residential distance from the asbestos cement plant was an important factor in mesothelioma risk (22). Modeled relative risk was 10.5 at the industrial site, 6.3 at 10 km from the site, and tapering to near unity at 12 km and beyond. These estimates were adjusted for occupational and para-occupational exposures. Another study of the same community, utilizing cumulative exposure estimates, showed monotonic increases in risk estimates for increasing categories of estimated cumulative environmental exposure, adjusted for occupational and para-occupational exposure (15). Exposure categories, primarily based on length of residence and distance from point source, were associated with the following statistically significant odds ratios relative to background exposure: 2.5 for 0.1 to <1 fiber/mL-years; 6.3 for 1 to <10 fiber/mL-years; and 14.4 for 10 or greater fiber/mL-years. Finally, a lung fiber burden study of eight non-occupational mesothelioma cases from the Casale Monferrato and Bari communities found 110,000 to 4,300,000 fibers per gram of dry lung (23). Lung fiber burden modeled on subject characteristics found significant main effects for residential distance from site and estimated cumulative environmental exposure. Thus, despite the confluence of occupational, para-occupational and environmental exposures in these communities with asbestos industry point sources, the evidence supports an increased risk for mesothelioma among people exposed environmentally, presumably via airborne routes.
The above studies, and similar studies in other locales showing decreasing mesothelioma risk with increasing distance for an asbestos plant (24-26), presume airborne exposures to neighborhoods from industrial point sources. Another pathway for environmental exposures from industrial sources relates to the distribution and use of asbestos-laden materials and waste products from local industry. We describe here two special case examples of such environmental exposure pathways and mesothelioma risk. Libby, MT, was previously mentioned as the source for mined vermiculite that was naturally contaminated with asbestos. More precisely the mining site was approximately seven miles from the town, but mined materials were distributed and used extensively in the community. For example, the mine waste material was used on the school running track, the community baseball field and in many residential gardens as a soil amendment (6). Screening studies have identified associations between non-occupational, environmental exposures to these materials and occurrence of pleural abnormalities (27,28). Despite the small size of the community, elevated SMRs for respiratory cancers have been observed (29), and 11 mesothelioma case reports among non-occupationally exposed residents were described (30). Only two of these 11 cases had a history of para-occupational exposure. The community of Wittenoom, Western Australia was established to support crocidolite mine and mill operations. Residents of the community, originally 1.5 km from the mine site then moved 11 km further, had experienced a high incidence of mesothelioma associated with para-occupational and environmental pathways (17,31). Mesothelioma mortality among specific sub-groups have been evaluated, including women with mixed environmental and para-occupational exposures (16) and Aboriginal people with mixed occupational and environmental exposures (32). Given the relatively small numbers of cases and the predominance of para-occupationally exposed cases among the women and occupationally exposed cases among the Aboriginal people, it is difficult to determine whether the mesothelioma risks associated with environmental pathways had occurred through past airborne exposure or direct contact with neighborhood tailings materials.
In summary, although the data are not entirely consistent, there is support for the association between airborne exposure to asbestos released from asbestos-related industrial point sources. In particular, the studies from the northwest Italy communities with asbestos cement plants provide a coherent argument for asbestos releases from these local facilities as the source of exposure for some of the observed non-occupational mesothelioma cases. Studies of other populations at larger scales and with multiple industrial sources being considered may indicate that exposure misclassification is too problematic for detecting robust associations with mesothelioma, a rare outcome outside the occupational setting. For similar reasons the association between neighborhood exposures from the use of local asbestos-laden waste products and risk of mesothelioma remains unclear.
Exposure to commercial asbestos-containing products
Beyond the resident populations that are para-occupationally or environmentally exposed to asbestos from local industrial sources, there are hundreds of asbestos-containing commercial products used throughout the world or already fixed in place. The products are varied and include automotive brakes, asbestos cement products, textiles, adhesives, insulation, ductwork parts and roofing and flooring materials. Asbestos products have been banned in over 50 countries, but most of the world’s population live in countries where commercial asbestos is still in use. Even in countries with bans, asbestos containing products remain fixed in place as a legacy of past use (33).
The linkage between exposure to these vastly distributed products with varying associated exposure potentials and risk of mesothelioma is difficult, if not impossible, to assess. Some mesothelioma case studies have been reported among people that may have been passively exposed when working in asbestos-insulated buildings [for review see (34)]. Likely of more concern is the active disturbance of asbestos-containing building materials. Indeed, the U.S. Centers for Disease Control and Preventions estimates that there are currently 1.3 million construction and industry workers in the United States that are being exposed to asbestos during renovation or demolition of old buildings (35). This concern of exposures related to home and building renovation or demolition activities is further highlighted by the historical widespread use of a particular attic insulation product. Zonilite Attic Insulation (ZAI) is an exfoliated vermiculite material that originated from the previously described asbestos-contaminated mining site in Libby, MT. ZAI was used extensively throughout the United States in home and commercial buildings. The product was received and/or processed at over 200 sites then locally distributed and sold. Estimates of the number of homes with ZAI range into the tens of millions (36), but these estimates are difficult to validate. The potential for asbestos exposure to homeowners or professional renovators and construction workers has been demonstrated in simulation studies showing that routine cleaning, maintenance, and remodeling activities that disturb ZAI can generate airborne amphibole asbestos exposures that exceed occupational exposure limits (37). Of interest, these exposure excursions were observed despite the fact that the bulk ZAI material often contains less the 0.1% asbestos, well below the 1% trigger level used by U.S. regulatory agencies to characterize building materials as asbestos-containing. No health studies have evaluated this exposure pathway and the association with mesothelioma or other asbestos-related diseases. Detection of such associations would be epidemiologically challenging given the low incidence of mesothelioma, the extensive geographic spread of potential home exposures, and the lack of knowledge regarding what homes contain ZAI or other asbestos-containing homebuilding or insulation products.
In Western Australia approximately 5% of incident mesothelioma cases between 1960 and 2008 were identified as cases where the only known exposure route was though home maintenance and/or renovation activity (38). Reported activities allocated to this exposure grouping included sanding asbestos cement walls, disrupting linoleum flooring or bathroom tiles, and transfer of asbestos cement sheeting as material for other building. The authors suggested that the home renovation exposure pathway is a rising contributor to mesothelioma rates. In support of this argument a recent survey of over 3,500 adults in a different Australian province, New South Wales, found that 44% of respondents had renovated their homes and half of those were do-it-yourself (DIY) home renovators (39). Over 50% of DIY renovators reported asbestos exposure during renovation. The survey also indicated strong likelihood for exposures to other household members, spouses and children, in those homes with reported renovation activity.
Overall, general population exposure to asbestos-containing commercial products is poorly understood and the associated health effects, including mesothelioma, are difficult to determine. Active disturbance of asbestos-containing building materials have the potential to generate health-relevant exposure concentrations. The observations in Western Australia suggest that mesothelioma cases attributed to DIY home renovation may be a growing concern in that region while occupationally-related mesothelioma cases are expected to be on the wane in the near future. The continued use of asbestos-containing building materials and other products as well as the fixed presence of asbestos-containing materials in countries where their use is now banned suggest that this is an area that warrants further investigation to understand future implications for mesothelioma risk.
Naturally occurring asbestos
Exposure to asbestos and related mineral fibers via unintended contact with natural geological formations is quite separate from the above categories that are related to commercial asbestos mining, processing and industrial applications. NOA includes asbestos-like fibrous minerals that occur naturally in rocks and soils. The concentration of NOA in these locations is typically lower than what is found in locations that have been exploited for mining. The NOA locations also do not typically meet regulatory definitions of asbestos or regulatory definitions for percent asbestos concentrations, and such definitions are too limiting for the purpose of assessing risks associated with exposure to NOA (2). NOA has been commonly found in populated areas and the various fibrous materials can be inhalation hazards when aerosolized through natural dust emissions and anthropogenic activities (40,41). Relevant anthropogenic activities are varied but can include soil disturbance related to construction and road building, recreational activities that generate dust, and harvesting of soils and rock for local use.
Over the last several decades several geographic foci with discoveries of NOA and concomitant observations of elevated mesothelioma rates have been described. NOA identified in these foci include tremolite and/or chrysotile in villages throughout Turkey, villages in northwest Greece, northern Corsica, the mountains of Cyprus, and New Caledonia; fluoro-edenite in Biancavilla, Sicily; erionite in the Cappadocian villages, Turkey; and crocidolite in a rural area of southwestern China (42-49). These reports shared several characteristics, albeit not universally, across the sites. First, these typically rural foci have shown mesothelioma incidence rates ranging from 100 to 800 times higher than global background rates. Further, mesothelioma incidence was shown to be inversely associated with distance from NOA sites in Turkey and New Caledonia (50,51). Second, as with several studies of para-occupational or environmental exposure to commercial asbestos, the male:female ratios were much lower, often close to one, than what had been observed in the occupational literature, and age at onset is often younger than what is observed in occupationally-exposed populations. Third, most investigations of these foci have identified specific exposure pathways that may be important for mesothelioma risk among these populations. For example, in the Turkey, Greece, Cyprus, Sicily and New Caledonia foci, local materials containing NOA were used for residential whitewashing, stucco or building stones. Case-control investigations in New Caledonia demonstrated that relevant exposures may not be limited to one pathway for mesothelioma risk, however, as one study indicated an association with pö (i.e., whitewash) (52) while another study found a strong association with proximity to roadways covered with a locally harvested serpentine material (51). The foci in Da-yao county in southern China offered a particularly complex exposure pattern with a crocidolite NOA used for road patching, stucco and painting as well as occupational exposures through the use of the material in a local stove building cottage industry (49). Finally, exposure investigations in these foci have yielded coherent evidence for these NOA contact pathways and risk of mesothelioma. For example, air sampling during high disturbance activities (e.g., sweeping or rubbing walls) in these homes with contaminated whitewash showed high fiber concentrations, and fiber burdens in lung tissue or bronchoalveolar lavage fluid of whitewash exposed people were similar to lung fiber burdens among occupationally exposed asbestos workers (53,54).
The above described foci of NOA and elevated mesothelioma rates have raised the question of whether our knowledge of NOA deposits can be translated to mesothelioma risk estimates for other populations living near such deposits. In California USA residences of mesothelioma cases and pancreatic cancer controls were mapped with respect to the distribution of ultramafic rocks, a known geologic source of amphibole NOA (55). Adjusting for probable occupational exposures, increasing distance (per 10 km) from ultramafic rock was associated with a 6.3% reduced risk of mesothelioma (95% CI: −10.5% to −1.8%). Despite the potential for exposure misclassification due to reliance on residence at time of diagnosis that may not reflect longest duration residence, this is an intriguing approach for estimating the impact of NOA on a large population when the effect estimate is small. In Dunn Country, North Dakota USA where erionite-containing gravel was used for paving local roads and other community facilities, parallels were inferred from the experiences of the Turkish erionite villages (56). Translation from the Turkish sites to future risk among these North Dakota populations is challenging given the different exposure pathways, yet the high carcinogenic potential of erionite argues for a precautionary approach when assessing exposure potential and mesothelioma risk. Finally, recent findings of NOA in southern Nevada USA prompted investigations of mesothelioma risk in this region that includes a large urban population and notable avenues for exposure to contaminated dust related to development activities, natural erosion and high disturbance recreational activities (40). Although clear indications of excess mesothelioma risk were not evident, observations of lower male:female ratios and younger age at onset among mesothelioma cases in other NOA-exposed populations offer additional avenues for evaluating epidemiological data (57). Geological studies in this area also have challenged common approaches for predicting the presence of NOA across large geographies. Such models have been based on the premise that most amphibole-containing formations are associated with ultramafic rock and deformation processes (i.e., folding, faulting, shearing or dilation) (58). The amphibole discovered in southern Nevada and northwest Arizona appear to be associated with granite plutons, suggesting that current models of NOA may under-predict opportunities for population exposure and associated health risk (59).
Thus, our understanding of mesothelioma risk associated with NOA exposures is limited. Several foci have showed that different types of NOA have been associated with elevated mesothelioma risk, and when compared to mesothelioma among occupationally-exposed populations these foci are often characterized by lower male:female ratios and younger age at onset. Another feature of these foci were well-characterized exposure pathways and coherent evidence of risk associated with these pathways. These foci offer important lessons when investigating the presence of NOA in specific, newly discovered exposure settings or across large geographies.
Summary
The vast majority of global mesothelioma cases are still attributed to asbestos-related occupations, but cases associated with environmental exposures will continue to be a measureable and growing component of disease risk. Asbestos bans and/or improved industrial hygiene and emission control practices in many of the para-occupational and environmental exposure settings referenced above may indicate that these pathways are of less future concern. However, global asbestos production and industrial use remains steady, and vulnerable populations will continue to be impacted by these exposure routes. Consumer contact with commercial asbestos-containing products will remain a viable exposure pathway, particularly where such products are fixed in place and easily disturbed through normal human activity such as home remodeling. The findings from Australia suggest that this pathway may be of growing importance for mesothelioma risk and an important area for health risk communication. Finally, opportunities for exposure contact with NOA are likely to grow as humans continue to develop and recreate in areas with previously undisturbed rock and as we learn more about the geological sources of asbestos. Further study in the newly discovered NOA locations with substantial populations at risk are needed to add to the evidence base for mesothelioma risk and prevention strategies related to NOA exposure.
Acknowledgements
The author is grateful to the many expert guides that have generously shared their time and experiences over the past several years, including Drs. Stephen Levin, Brad Black, Jaime Szeinuk, Albert Miller, Jean Pfau, Claudia Henschke, David Yankelevitz and Raja Flores.
Funding: This work was supported, in part, by a grant from the U.S. Centers for Disease Control and Prevention (TS09-001). Additional support for Dr. Noonan provided by National Institute of General Medical Sciences (COBRE P30GM103338).
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- U.S. Geological Survey. Mineral commodity summaries. 2016.
- Baumann F, Ambrosi JP, Carbone M. Asbestos is not just asbestos: an unrecognised health hazard. Lancet Oncol 2013;14:576-8. [Crossref] [PubMed]
- Sahmel J, Barlow CA, Simmons B, et al. Evaluation of take-home exposure and risk associated with the handling of clothing contaminated with chrysotile asbestos. Risk Anal 2014;34:1448-68. [Crossref] [PubMed]
- Goswami E, Craven V, Dahlstrom DL, et al. Domestic asbestos exposure: a review of epidemiologic and exposure data. Int J Environ Res Public Health 2013;10:5629-70. [Crossref] [PubMed]
- Roggli VL, Sharma A, Butnor KJ, et al. Malignant mesothelioma and occupational exposure to asbestos: a clinicopathological correlation of 1445 cases. Ultrastruct Pathol 2002;26:55-65. [Crossref] [PubMed]
- Noonan CW, Conway K, Landguth EL, et al. Multiple pathway asbestos exposure assessment for a Superfund community. J Expo Sci Environ Epidemiol 2015;25:18-25. [Crossref] [PubMed]
- Donovan EP, Donovan BL, McKinley MA, et al. Evaluation of take home (para-occupational) exposure to asbestos and disease: a review of the literature. Crit Rev Toxicol 2012;42:703-31. [Crossref] [PubMed]
- Vianna NJ, Polan AK. Non-occupational exposure to asbestos and malignant mesothelioma in females. Lancet 1978;1:1061-3. [Crossref] [PubMed]
- Bianchi C, Bianchi T. Malignant pleural mesothelioma in Italy. Indian J Occup Environ Med 2009;13:80-3. [Crossref] [PubMed]
- Miller A. Mesothelioma in household members of asbestos-exposed workers: 32 United States cases since 1990. Am J Ind Med 2005;47:458-62. [Crossref] [PubMed]
- Howel D, Arblaster L, Swinburne L, et al. Routes of asbestos exposure and the development of mesothelioma in an English region. Occup Environ Med 1997;54:403-9. [Crossref] [PubMed]
- Rake C, Gilham C, Hatch J, et al. Occupational, domestic and environmental mesothelioma risks in the British population: a case-control study. Br J Cancer 2009;100:1175-83. [Crossref] [PubMed]
- Magnani C, Agudo A, Gonzalez CA, et al. Multicentric study on malignant pleural mesothelioma and non-occupational exposure to asbestos. Br J Cancer 2000;83:104-11. [PubMed]
- Ferrante D, Bertolotti M, Todesco A, et al. Cancer mortality and incidence of mesothelioma in a cohort of wives of asbestos workers in Casale Monferrato, Italy. Environ Health Perspect 2007;115:1401-5. [PubMed]
- Ferrante D, Mirabelli D, Tunesi S, et al. Pleural mesothelioma and occupational and non-occupational asbestos exposure: a case-control study with quantitative risk assessment. Occup Environ Med 2016;73:147-53. [Crossref] [PubMed]
- Reid A, Heyworth J, de Klerk N, et al. The mortality of women exposed environmentally and domestically to blue asbestos at Wittenoom, Western Australia. Occup Environ Med 2008;65:743-9. [Crossref] [PubMed]
- Reid A, Berry G, de Klerk N, et al. Age and sex differences in malignant mesothelioma after residential exposure to blue asbestos (crocidolite). Chest 2007;131:376-82. [Crossref] [PubMed]
- Goldberg M, Imbernon E, Rolland P, et al. The French National Mesothelioma Surveillance Program. Occup Environ Med 2006;63:390-5. [Crossref] [PubMed]
- Horton DK, Bove F, Kapil V. Select mortality and cancer incidence among residents in various U.S. communities that received asbestos-contaminated vermiculite ore from Libby, Montana. Inhal Toxicol 2008;20:767-75. [Crossref] [PubMed]
- Corfiati M, Scarselli A, Binazzi A, et al. Epidemiological patterns of asbestos exposure and spatial clusters of incident cases of malignant mesothelioma from the Italian national registry. BMC Cancer 2015;15:286. [Crossref] [PubMed]
- Mensi C, Riboldi L, De Matteis S, et al. Impact of an asbestos cement factory on mesothelioma incidence: global assessment of effects of occupational, familial, and environmental exposure. Environ Int 2015;74:191-9. [Crossref] [PubMed]
- Maule MM, Magnani C, Dalmasso P, et al. Modeling mesothelioma risk associated with environmental asbestos exposure. Environ Health Perspect 2007;115:1066-71. [Crossref] [PubMed]
- Barbieri PG, Mirabelli D, Somigliana A, et al. Asbestos fibre burden in the lungs of patients with mesothelioma who lived near asbestos-cement factories. Ann Occup Hyg 2012;56:660-70. [PubMed]
- Tarres J, Alberti C, Martinez-Artes X, et al. Pleural mesothelioma in relation to meteorological conditions and residential distance from an industrial source of asbestos. Occup Environ Med 2013;70:588-90. [Crossref] [PubMed]
- Berry M. Mesothelioma incidence and community asbestos exposure. Environ Res 1997;75:34-40. [Crossref] [PubMed]
- Kurumatani N, Kumagai S. Mapping the risk of mesothelioma due to neighborhood asbestos exposure. Am J Respir Crit Care Med 2008;178:624-9. [Crossref] [PubMed]
- Peipins LA, Lewin M, Campolucci S, et al. Radiographic abnormalities and exposure to asbestos-contaminated vermiculite in the community of Libby, Montana, USA. Environ Health Perspect 2003;111:1753-9. [Crossref] [PubMed]
- Szeinuk J, Noonan CW, Henschke CI, et al. Pulmonary abnormalities as a result of exposure to Libby amphibole during childhood and adolescence-The Pre-Adult Latency Study (PALS). Am J Ind Med 2017;60:20-34. [Crossref] [PubMed]
- Agency for Toxic Substances and Disease Registry. Mortality in Libby, Montana (1979 - 1998) 2002.
- Whitehouse AC, Black CB, Heppe MS, et al. Environmental exposure to Libby Asbestos and mesotheliomas. Am J Ind Med 2008;51:877-80. [Crossref] [PubMed]
- Hansen J, de Klerk NH, Musk AW, et al. Environmental exposure to crocidolite and mesothelioma: exposure-response relationships. Am J Respir Crit Care Med 1998;157:69-75. [Crossref] [PubMed]
- Franklin P, Reid A, Olsen N, et al. Incidence of malignant mesothelioma in Aboriginal people in Western Australia. Aust N Z J Public Health 2016;40:383-7. [Crossref] [PubMed]
- Ramazzini C. Asbestos is still with us: Repeat call for a universal ban. Am J Ind Med 2011;54:168-73. [Crossref] [PubMed]
- Goldberg M, Luce D. The health impact of nonoccupational exposure to asbestos: what do we know? Eur J Cancer Prev 2009;18:489-503. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). Malignant mesothelioma mortality--United States, 1999-2005. MMWR Morb Mortal Wkly Rep 2009;58:393-6. [PubMed]
- Spear TM, Hart JF, Spear TE, et al. The presence of asbestos-contaminated vermiculite attic insulation or other asbestos-containing materials in homes and the potential for living space contamination. Journal of Environmental Health 2012;75:24-9. [PubMed]
- Ewing WM, Hays SM, Hatfield R, et al. Zonolite attic insulation exposure studies. Int J Occup Environ Health 2010;16:279-90. [Crossref] [PubMed]
- Olsen NJ, Franklin PJ, Reid A, et al. Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation. Med J Aust 2011;195:271-4. [Crossref] [PubMed]
- Park EK, Yates DH, Hyland RA, et al. Asbestos exposure during home renovation in New South Wales. Med J Aust 2013;199:410-3. [Crossref] [PubMed]
- Buck B, Goossens D, Metcalf R, et al. Naturally occurring asbestos: Potential for human exposure, southern Nevada, USA. Soil Science Society of America Journal 2013;77:2192-204. [Crossref]
- Carbone M, Kanodia S, Chao A, et al. Consensus Report of the 2015 Weinman International Conference on Mesothelioma. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer 2016;11:1246-62.
- Bayram M, Bakan ND. Environmental exposure to asbestos: from geology to mesothelioma. Curr Opin Pulm Med 2014;20:301-7. [Crossref] [PubMed]
- Constantopoulos SH, Malamou-Mitsi VD, Goudevenos JA, et al. High incidence of malignant pleural mesothelioma in neighbouring villages of Northwestern Greece. Respiration 1987;51:266-71. [Crossref] [PubMed]
- Constantopoulos SH. Environmental mesothelioma associated with tremolite asbestos: lessons from the experiences of Turkey, Greece, Corsica, New Caledonia and Cyprus. Regul Toxicol Pharmacol 2008;52:S110-5. [Crossref] [PubMed]
- McConnochie K, Simonato L, Mavrides P, et al. Mesothelioma in Cyprus: the role of tremolite. Thorax 1987;42:342-7. [Crossref] [PubMed]
- Luce D, Brochard P, Quenel P, et al. Malignant pleural mesothelioma associated with exposure to tremolite. Lancet 1994;344:1777. [Crossref] [PubMed]
- Bruno C, Tumino R, Fazzo L, et al. Incidence of pleural mesothelioma in a community exposed to fibres with fluoro-edenitic composition in Biancavilla (Sicily, Italy). Ann Ist Super Sanita 2014;50:111-8. [PubMed]
- Carbone M, Emri S, Dogan AU, et al. A mesothelioma epidemic in Cappadocia: scientific developments and unexpected social outcomes. Nat Rev Cancer 2007;7:147-54. [Crossref] [PubMed]
- Luo S, Liu X, Mu S, et al. Asbestos related diseases from environmental exposure to crocidolite in Da-yao, China. I. Review of exposure and epidemiological data. Occup Environ Med 2003;60:35-41; discussion 41-2. [Crossref] [PubMed]
- Bayram M, Dongel I, Bakan ND, et al. High risk of malignant mesothelioma and pleural plaques in subjects born close to ophiolites. Chest 2013;143:164-71. [Crossref] [PubMed]
- Baumann F, Maurizot P, Mangeas M, et al. Pleural mesothelioma in New Caledonia: associations with environmental risk factors. Environ Health Perspect 2011;119:695-700. [Crossref] [PubMed]
- Luce D, Bugel I, Goldberg P, et al. Environmental exposure to tremolite and respiratory cancer in New Caledonia: a case-control study. Am J Epidemiol 2000;151:259-65. [Crossref] [PubMed]
- Luce D, Billon-Galland MA, Bugel I, et al. Assessment of environmental and domestic exposure to tremolite in New Caledonia. Arch Environ Health 2004;59:91-100. [Crossref] [PubMed]
- Dumortier P, Coplu L, de Maertelaer V, et al. Assessment of environmental asbestos exposure in Turkey by bronchoalveolar lavage. Am J Respir Crit Care Med 1998;158:1815-24. [Crossref] [PubMed]
- Pan XL, Day HW, Wang W, et al. Residential proximity to naturally occurring asbestos and mesothelioma risk in California. Am J Respir Crit Care Med 2005;172:1019-25. [Crossref] [PubMed]
- Carbone M, Baris YI, Bertino P, et al. Erionite exposure in North Dakota and Turkish villages with mesothelioma. Proc Natl Acad Sci U S A 2011;108:13618-23. [Crossref] [PubMed]
- Baumann F, Buck BJ, Metcalf RV, et al. The Presence of Asbestos in the Natural Environment is Likely Related to Mesothelioma in Young Individuals and Women from Southern Nevada. J Thorac Oncol 2015;10:731-7. [Crossref] [PubMed]
- Van Gosen B. The geology of asbestos in the United States and its practical applications. Environmental and Engineering Geoscience 2007;13:55-68. [Crossref]
- Metcalf R, Buck B. Genesis and health risk implications of an unusual occurrence of fibrous NaFe3+-amphibole. Geology 2015;43:63-6. [Crossref]


