Long-term effects of video capsule endoscopy in the management of obscure gastrointestinal bleeding
Introduction
Obscure gastrointestinal bleeding (OGIB) is defined as persisting and/or recurrent gastrointestinal (GI) bleeding of unidentified source after negative bidirectional endoscopic evaluation (1). OGIB can be further categorized into occult and overt bleeding (2). While occult OGIB occurs in the setting of a positive fecal occult blood test (FOBT) or iron deficiency anemia (IDA), overt OGIB is the clinically evident manifestation of GI bleeding as melena or hematochezia (2,3). The advent of video capsule endoscopy (VCE), revolutionized the approach and management of patients with small bowel diseases and particularly with OGIB (4). Apart from VCE, a number of other sophisticated diagnostic innovations have emerged, allowing identification of a small intestine bleeding source in the majority of patients with OGIB. As a result, it has been recently recommended that the term OGIB should be preserved only for those patients remaining undiagnosed after meticulous examination of the entire GI tract (5). At present, VCE holds the pivotal role in the optimal evaluation strategy for patients with OGIB (6). It is superior to push enteroscopy, barium contrast radiology, computed tomography and magnetic resonance imaging for diagnosing clinically significant small bowel lesions (7-9). At the same time, it achieves complete small bowel examination in about 80–85% of the cases and demonstrates equivalent diagnostic yield to device-assisted enteroscopy (10-12), retaining its noninvasive nature, patient tolerability and excellent safety profile (13). VCE not only accurately determines the preferred route for DBE insertion (oral vs. anal) but also selects those most likely to benefit from this procedure (12,13). Thus, recent guidelines of the European Society of Gastrointestinal Endoscopy (ESGE) endorse VCE as the first-line small bowel investigative modality in patients with OGIB (14). However, the clinical impact of this investigation in the long-term has not received the same attention, yet. Important issues such as the effectiveness in prediction and assessment of re-bleeding risk, need for ongoing treatment i.e. blood transfusions and hospitalization rate remain underrated, since most of the published literature has so far focused on short term endoscopic results. The aim of this paper is to review the existing data regarding the long-term clinical outcomes of VCE in the management of patients with OGIB.
Methods
We conducted a comprehensive literature review, aiming to identify all papers published in English from January 2002 to October 2016 regarding the long-term effects of VCE small bowel investigation in the management of patients with OGIB. The search was carried out in the PubMed electronic database with the following key words: “capsule endoscopy”, “obscure GI bleeding”, “long-term” and “effects”, alone or with various combinations. We favored the term “obscure gastrointestinal bleeding” compared to “small bowel bleeding” or “mid-gut bleeding” to prevent missing references. Subsequently, a manual search of the references cited in the key articles was carried out. Each result was crosschecked by two authors (GT, PG) to achieve a maximum completeness of the reports chosen for inclusion; while final decision in case of disagreement with respect to the appropriateness of an article, was reached by the senior author (KT). Only relevant articles comprising long-term follow-up data—arbitrary defined as longer ≥12 months after index VCE—were considered eligible for review. We identified 61 relevant titles initially; 34 original studies are discussed (Table S1 summarizes the main characteristics of the included studies). Data from meta-analyses and systematic reviews are also discussed to further highlight the context of the study. The search strategy applied is depicted in Figure 1.
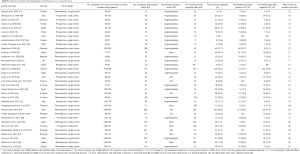
Full table
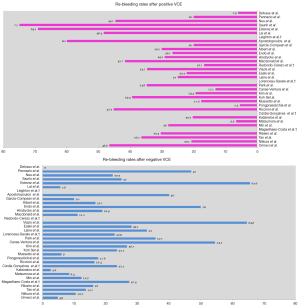
Re-bleeding rate after index VCE small bowel examination
The long-term clinical implications of VCE small bowel examination have been at the focal point of several studies, aiming to determine its efficacy in prediction and assessment of re-bleeding risks. Differences in reported clinical outcomes derive from the significant heterogeneity among studies regarding design, number of enrolled patients, uneven length of follow-up, lack of standardized subsequent management or follow-up modality, inter-observer variability regarding VCE findings interpretation (ulcer/erosions can be interpreted either as positive or negative finding) and single-center experience (Figure 2).
Accumulating evidence has highlighted the impact of a confirmatory diagnosis accomplished by a VCE examination and its therapeutic and prognostic implications. On the other hand, a negative VCE exam provides reassurance against the likelihood of re-bleeding since its negative predictive value (NPV) is high, favoring a wait and watch approach (3,14), in the short term. However, data on the long-term outcomes of patients with a negative VCE are scant. Undoubtedly, VCE may miss significant lesions due to its inherent imperfections (15,16). Although studies have examined objective measures such as whether a negative test leads to other tests or therapeutic interventions, the question about the long-term natural course of a negative VCE remains controversial.
More than a decade ago, Delvaux et al. (17) in a well-designed, 12-month follow-up prospective trial of 44 patients showed that a negative VCE achieves a NPV of 100% for relevant small intestine lesions. This implies that a normal examination may not always expedite a diagnosis; however, it allows the physician to quit a certain line of investigations and advocate an expectant approach. One of the first studies trying to assess the cumulative re-bleeding rate of OGIB was published in 2005 by Neu et al. (18). In this prospective, multicenter study, re-bleeding occurred more frequently in patients with positive than negative VCE (44.7% vs. 22.2%) after 13-month follow-up period. One year later, Lai et al. (19) presented a pioneer study where 49 patients diagnosed with OGIB were followed for a mean time of 19 months. Those with positive index VCE exam had a significantly higher re-bleeding rate compared to those with negative (48.4% vs. 5.6%, P=0.03). Surprisingly, about two-thirds (64.5%) of the patients with a positive VCE did not receive any form of subsequent treatment. Hence, one could argue that the long-term re-bleeding rate of this group of patients could have been lower after treatment application. However, it should be emphasized that DBE at that time was not widely available and that in real world clinical practice further interventions are not routinely applied to all subjects, even today. Moreover, this gave the authors the opportunity to observe the natural history of OGIB, identifying angiodysplasia as a significant predictor of re-bleeding, whereas ulcers or erosions re-bled seldom (20). In 2008, Macdonald et al. (21) reported the results of a retrospective study assessing the long-term efficacy of VCE in 42 patients with OGIB, with a mean follow-up of 17 months. A statistical difference in re-bleeding rate between patients with positive and negative VCE findings was found (41.7% vs. 11.1%, P<0.01). Their results indicate that VCE has high specificity and NPV in the long-term; thus, further interventions in case of negative VCE can be withheld. Although well designed, the aforementioned studies attracted criticism mainly due to the small number of enrolled patients. To overcome this limitation, a multicenter, retrospective study from Germany assessed for the first time the long-term efficacy of VCE in a considerably large (n=240) number of patients (22). A re-bleeding rate of about 30% after positive VCE was reported, while re-bleeding rate was 16.7% in the cases with negative findings, further highlighting the validity of VCEs NPV.
Thereafter, results from one Greek and one American center came to light. The former, in a prospective study investigating the role of second-look VCE, reported that 65% of patients with a negative initial VCE continued to have OGIB after a mean follow-up period of 24 months (23). Laine and colleagues—in the only randomized controlled study ever conducted on this subject—demonstrated that re-bleeding rate was 33% in patients with normal VCE and 25% in positive VCE; underlining the significance of performing further examination to reveal the bleeding source in negative VCE cases (24). Taken together, these studies demonstrated somewhat conflicting results regarding the role of VCE; however, they should be seen critically since the follow-up period was relative short.
Trying to provide further clarifications, studies including larger populations with longer follow-up period were designed. Riccioni et al. (25) conducted a retrospective single center study of 696 OGIB cases with follow-up period averaging 24 months. The re-bleeding rate was higher in patients with positive VCE than in cases with negative study (45.2% vs. 16.4%, P=0.001), thus concluding that a negative VCE correlates to low long-term re-bleeding rate and further invasive investigations could be deferred. A recently published paper reported the results of a prospective multicenter study in referral and inexperienced in advanced therapeutic enteroscopy centers; thus, offering an accurate simulation of real world clinical practice (26). Authors observed re-bleeding in 16 (20.3%) patients with positive and only in a single (2.6%) patient with negative VCE, confirming the findings by Lai et al. (19). Similar results were also obtained by a retrospective European study presenting one of the largest series of patients (n=173) with OGIB with follow-up period longer than 27 months. A higher re-bleeding rate after positive VCE was reported in comparison with negative VCE (30.4% vs. 16%, P=0.02) (27). Outside of Europe, a recent retrospective cohort study from China, evaluated 339 patients over a follow-up of 48 months. Re-bleeding rate in cases with positive VCE was significantly higher than that in those with negative VCE exam (36.5% vs. 13.7%, P=0.0001) (28). Further insight into clinical outcomes after VCE is provided by a contemporary multi-center study that investigated a large sample of patients (n=320) (29): positive index VCE was associated with a higher re-bleeding rate than negative index exam (20.7% vs. 10.5%, P=0.024). Long-term outcomes were studied further by a retrospective follow-up study of 139 patients undergoing VCE for OGIB in Turkey. A positive VCE study was associated with significantly higher re-bleeding risk in the long-term compared to negative one (46.6% vs. 4.8%, P=0.001) (30). In an effort to optimize risk definition, authors from a large retrospective European trial followed patients with negative index VCE exam for a period of 5 years. Remarkably, the majority of re-bleeding episodes (81.3%) occurred within the first 2 years after VCE examination, while the cumulative risk of re-bleeding raised from 12.9% at 1, to 25.6% at 3 and to 31.5% at 5 years, respectively (31); this finding being verified by other authors (29). In keeping with the findings of previous reports (25,32), the median time until first re-bleeding event was 15 months, underlying the value of patient monitoring for at least the first 2 years post negative VCE exam and perhaps later on, as the interquartile range for the time lag to re-bleeding was between 2 and 33 months (31).
Unlike the previous studies, no differences in re-bleeding rates according to VCE results were reported in studies arising from Eastern countries (32-37). Endo and associates (33) found significantly higher rate of re-bleeding in patients with negative VCE, compared to those with significant findings (50% vs. 12%). Authors concluded that VCE negative patients should undergo regular follow-up, whilst being mindful that the bleeding may not originate from the small bowel. Park et al. (34), in a Korean single center retrospective analysis of 57 consecutive patients followed for 31.7 months, found a comparable re-bleeding rate between patients with positive and negative VCE exams (34.8% vs. 35.7%, P=0.989), suggesting that this might be due to a 19.6% miss rate of VCE for small bowel lesions, in the setting of equal small-bowel transit time (SBTT) during OGIB between re-bleeders and non-re-bleeders (31). In another Korean study, Kim et al. (35) reported a re-bleeding rate of 26.7% in patients with negative VCE without subsequent treatment and 21.2% in positive VCE without specific treatment, respectively (P=0.496). Moreover, Koh et al. (32) also failed to reveal any difference in the re-bleeding rate between patients with positive and negative VCE exams (39.4% vs. 23.5%, P=0.205). In a retrospective study originating from Thailand, the re-bleeding rate after negative VCE study was higher compared to that after positive examination (18% vs. 5.4%, P=0.08) (36). Authors proposed that the low re-bleeding rate directly links to the etiology of the underlying disease - spontaneously resolving small bowel ulcer, being the most common lesion detected in the study that does not recur. Of note, the final analysis showed that all re-bleeding episodes in patients with negative VCE occurred due to non-small bowel lesions; reinforcing VCE`s ability to efficiently rule out small bowel source of OGIB (17,38). Finally, a similar pattern of re-bleeding was reported by Min et al. (37) in a large nationwide, prospective, multicenter cohort study with mean follow-up period of 38.7 months. Re-bleeding rate was the similar regardless of VCE result or treatment application. Small bowel ulcer—the predominant finding—did not show different re-bleeding rate compared with negative findings. On the contrary, angiodysplasia—although less frequent—showed a higher re-bleeding rate.
A number of potential reasons explaining this discordance in long-term outcomes reported in the latter studies could be cited. Firstly, investigation of potential differences in long-term re-bleeding rate after negative VCE was not the primary endpoint of early Western studies (17,21,39,40); thus, questions about their power are raised. Beyond the small size of the examined population, the study design may also be considered as a caveat: prospective studies reported significantly lower re-bleeding rate after negative VCE compared to retrospective trials (9). An issue that should be also highlighted is the follow-up period. Studies reporting very low re-bleeding rates had relative short follow-up (17,19,21,36,40,41). Contrariwise, Korean studies enrolled relatively more subjects with longer follow-up, exceeding 2 years (32,34,35,37). This possibly allowed Park et al. (34) to display a re-bleeding rate of 35.7% during the 32 months follow-up, while Koh et al. (32) showed that more than half of the patients suffered bleeding recurrence more than 1 year after the initial episode with the maximum time to re-bleeding being 24 months post-procedurally. Finally, an alternative explanation could be speculated on the basis of the ethnicity of the recruited population. More precisely, angiodysplasia is the most prevalent finding among Western reports, whereas ulcer or erosions is the culprit lesion among Asians. This is in line with a previous meta-analysis of DBE studies including 5,268 OGIB cases, that identified inflammatory (37.6%) and vascular (65.9%) lesions as the most common findings in Eastern and Western countries (Europe, North America and Australia), respectively (42). Reasons behind this striking difference are poorly understood. Angiodysplasia often has a multifocal pattern, elusive natural course and carries a high potential for re-bleeding since half of the cases re-bled within 3 years despite ongoing usage of effective treatment modalities i.e. argon plasma coagulation (2,43). Consequently, re-bleeding risk seems to be directly linked to the underlying cause rather than VCE exam results or therapeutic treatment application.
The re-bleeding rate after negative VCE and the impact on long-term follow-up has been addressed in a very recent meta-analysis, including 26 studies (3,657 patients) (9). Both prospective and retrospective studies were included resulting in significant heterogeneity. In addition, most of the studies were focused on outcomes after positive VCE, thus understating outcomes after negative examination. Investigators demonstrated that the overall pooled rate of re-bleeding after negative VCE exam was significantly lower compared to that after positive examination (0.19 vs. 0.29, P<0.001). Regarding the study’s primary outcome, the overall odds ratio (OR) for re-bleeding was lower after a negative VCE exam as compared to a positive one (0.59; 95% CI, 0.37–0.95; P<0.001); this finding being further enhanced when studies with short follow-up were included. No difference between the re-bleeding rates with respect to capsule model used, type of OGIB or specific treatment was noted, meaning that conservative approach is suitable for these patients. Notably, OR for re-bleeding rate after negative VCE increased with time; suggesting that the “protective effect” of a negative VCE on forthcoming re-bleeding episodes applies over a certain amount of time, estimated as the first 2 years post-procedurally. However, this beneficial effect seems to fade on the long-term as new bleeding sources (mainly angiodysplasias) may rise. Moreover, the possibility of false negative VCE should not be ignored (44). Potential bleeding recurrence manifesting as change from occult to overt presentation or ≥4 g/dL drop in hemoglobin, should prompt a “second-look” VCE investigation as acknowledged by current ESGE guidelines (14).
Impact on subsequent therapeutic strategies and clinical outcomes
A VCE study with significant findings will lead to specific treatments application, i.e., device-assisted enteroscopy preferably, aiming to treat identified lesions. Nonetheless, VCE will not achieve to detect the bleeding lesion in a sizeable proportion (up to one third) of patients with OGIB; constituting long-term management of those patients a clinical challenge. Several reviews and consensus recommendations support conservative approach when VCE is non-diagnostic and evidence of ongoing bleeding is lacking (3,45). Still, even recent guidelines were not fully based on long-term re-bleeding data (14).
The prospective, multicenter study by Pennazio et al. (46), aimed to elucidate whether VCE impacts the indication for further diagnostic procedures and clinical outcome of OGIB. VCE results affected patients’ outcome directing towards diagnostic techniques that resolved bleeding in 86.9% of cases; these results being verified by other concurrent publications (47). Albert et al. (22) found that VCE results determined the therapy in 66% of the cases and led to an alteration in management in 32.3% of the cases. In accordance, Endo et al. (33) found that the re-bleeding rate of patients who underwent therapeutic intervention after positive VCE small bowel exam was significantly lower (9.5% vs. 40.0%, P=0.046) than that of those who did not undergo treatment. Consistently, Park et al. (34) demonstrated that when specific treatment after VCE (DBE not included) was applied, a significant decrease in re-bleeding was noted (HR: 0.111; 95% CI, 0.013–0.980; P=0.043). Thus, implementation of an aggressive management to detect and treat the underlying bleeding lesion is encouraged. This finding was also supported by other studies (19,34,48,49). In the Ribeiro et al. study (27), more than half of patients with positive VCE received specific treatment, thus decreasing the re-bleeding risk. Angiodysplasias had re-bleeding rates of 24% if no treatment had been applied and 8% if a therapeutic intervention was applied. Taking these observations into account, it is evident that VCE accurately identifies those patients who are likely to benefit from subsequent interventions.
Re-bleeding rates did not vary after treatment across the cohort of Macdonald et al. (21); however, the study’s subgroup which received treatment comprised only nineteen patients which seems inadequate to support a conclusion. In a trial of 260 patients, outcomes appeared to improve after VCE: patients experienced fewer bleeding events and interventions per month following VCE than those they did pre-VCE (50). However, these results are limited by the variable interval of patient follow-up pre- and post-VCE, as in any retrospective study. Meanwhile, Viazis et al. (23) reported that VCE affects long-term outcome (i.e., resolution of bleeding) in patients with positive VCE (65.2% vs. 35.4% of negative VCE group), since these patients are prone to undergo aggressive interventions. Angiodysplasia was the most common finding among patients with positive VCE (70%) and the majority of them received some mode of treatment leading to bleeding resolution (69%). Esaki et al. (51) in their trial, performed therapeutic interventions in 28 of 36 patients with positive VCE findings. The reported re-bleeding rate (10.7%) was equivalent to those in previous studies (6–10%), implying that use of anti-coagulation therapy or type of therapy may pose a certain effect. Similarly, the re-bleeding rate in the subgroup of patients receiving specific treatments was significantly lower (22.4% vs. 34.9%, P=0.007) compared to that of those with nonspecific treatments (28).
On the contrary, augmentation in diagnostic yield with the use of VCE does not improve outcome in patients with OGIB according to a prospective, randomized control trial (24). Koh et al. (32) in their multivariate analysis also found that specific treatment does not lead to reduced risk of re-bleeding; acknowledging a limited role of VCE in the clinical outcome of patients with OGIB. However, several limitations can be noted i.e. retrospective, single center study, DBE unavailability or incomplete VCE examinations dictating careful data interpretation. Similarly, Min et al. (37) reported that application of interventional treatment even in patients with angiodysplasia failed to decrease re-bleeding after VCE; however, its value should not be underestimated. Katsinelos et al. (26) claimed that positive VCE investigations led to therapeutic decisions (endoscopic, surgical, pharmaceutical) that decreased re-bleeding of patients as opposed to that of patients with negative exams (9% vs. 44%, P<0.001) and improved the clinical condition in 71.4% of them. Hindryckx et al. (39) in a retrospective ‘‘real-life’’ analysis also reported favorable outcomes in 61 of 92 patients after VCE guided therapy during a mean follow-up period of 635.5 days. However, no difference between the VCE-positive (33/55, 60.0%) and VCE-negative group (28/37, 75.7%) was noted regarding condition outcome. Finally, in a recent retrospective study, VCE was associated with a favorable outcome in the majority of patients (52). Surprisingly, treatment application did not correlate with a higher (20.5% vs. 36.4%, P=0.8) resolution re-bleeding rate after specific or nonspecific treatment, respectively; signaling the potential role of angiodysplasia—the most commonly encountered finding—in re-bleeding risk. It was therefore suggested, that VCE does not actually influence long-term patient outcomes. At best, it can determine which patients are most likely to benefit from subsequent therapeutic work-up. Patients with positive VCE necessitate endoscopic interventions for effective treatment and DBE has been shown to provide significant aid in patients with small bowel lesions or those who are at high risk for re-bleeding despite a non-diagnostic VCE (25,35). In a meta-analysis of seven studies, the diagnostic yield for DBE after negative VCE for OGIB was 27.5% (95% CI, 16.7–37.8%) (12), while VCE followed by DBE has been established as an effective strategy for investigating OGIB and particularly to confirm a negative VCE examination (53). Hence, DBE does not compete to VCE; it has a rather complementary role. Conversely, the risk of re-bleeding in patients with negative VCE is sufficiently low; thus, supplementary investigations could be postponed until further clinical indications arise.
With specific regard to other clinical outcomes such as blood transfusions requirements, GI procedures, hospitalization duration and hemoglobin levels, a preliminary prospective analysis of 20 patients undergoing VCE for OGIB showed significant reduction over 1 year period (54). Additional data supported that patients with positive VCE require longer hospitalization as well as higher number of blood units transfused than those with negative VCE exams (19,21,25,50,55). Even on the occasion of a re-bleeding episode, patients with negative index VCE require neither hospital admission nor blood transfusions (25). Moreover, a higher number of blood units transfused before VCE correlates with an increased re-bleeding risk, possibly indicating presence of serious GI tract lesion (27). Nevertheless, VCE remains a purely diagnostic test and the presented improvements in bleeding parameters cannot be directly attributed to the examination: VCE at best can direct clinicians towards the most suitable therapeutic measures.
In terms of economic impact, management of OGIB patients entails considerable expense (56). In the era of financial crisis (57), fiscal austerity measures have been imposed throughout Europe posing negative impact on public health care systems (58). At the same time, while VCE indications were optimized, the number of studies conducted has decreased significantly in a financial environment like this (59). Therefore, in many countries the need for an examination in the diagnostic algorithm of OGIB that combines the high diagnostic yield with cost-effectiveness is more pertinent than ever. VCE has been shown to be an economically sound testing strategy (56,60). Considering its high NPV, patients with negative VCE reasonably undergo no further diagnostic work-up; minimizing procedural costs on the long-term (55,56). Furthermore, at the time of re-bleeding expenditures of these patients are negligible since hospital admission or blood transfusions seem to be unnecessary (25). DBE is more cost-effective since it does not only provide therapeutic potential but it also ameliorates additional costs regarding further investigations (56). However, one should have in mind that DBE imposes a significant burden to the endoscopist, is not universally available and also hinders a considerable risk for complications (12). A VCE-directed DBE leads to better outcomes in the long-term because of lower complications rate and reduction of endoscopic resources used (61).
Predictive factors for re-bleeding after index VCE
A few, albeit significant clinical factors, i.e., Hb lower than 6.7 g/dL, more than 5 blood units transfused and positive VCE exam have been identified by the initial studies as determinants of further bleeding events (18,46). Among others, the potential role of anticoagulants has been pinpointed by many studies (Table 1). More precisely, Macdonald and colleagues reported that warfarin use correlates to high re-bleeding risk overall; however, their sample was small and multivariate analysis was not performed (21). Although Kim et al. (35) analyzed only patients with negative VCE and those who did not receive further treatment, they found that concomitant use of warfarin correlates (P<0.001) to a high re-bleeding risk. Accordingly, Koh et al. (32) identified anticoagulation therapy as an independent risk factor for re-bleeding (HR 5.019; 95% CI, 1.560–16.145; P=0.007). However, the number of re-bleeding events analyzed was in both studies small (16/60 and 12/51, respectively); thus, generalizability of these results is limited. In the study by Min et al. (37), 66/116 patients who discontinued anticoagulant medication after VCE demonstrated lower re-bleeding rate; interestingly, use of anticoagulants before and after VCE was not associated with re-bleeding, overall. Similar to previous reports, Cúrdia Gonçalves et al. (62) found only marginal association between anticoagulants use and re-bleeding episode, while another study identified anticoagulant use as predictor of future re-bleeding events (HR: 3.9; 95% CI, 1.5–9.9; P=0.004) (31). However, since all these studies were powered only for the primary endpoint and investigation for predictors of re-bleeding were regarded merely a secondary one, the possibility of a type II error cannot be excluded. The multivariate regression analysis of a well-designed study combining large number of VCE examinations along with long follow-up (more than 45 months) revealed that use of anticoagulants predicted a high risk of re-bleeding (28). Finally, Niikura et al. (29), in a large cohort indicated warfarin use as one of the five potential risk factors (female gender, cirrhosis, warfarin use, overt bleeding, positive VCE) for re-bleeding. Undoubtedly, confirmation or rejection of these results requires more evidence since relevant prospective data are lacking.
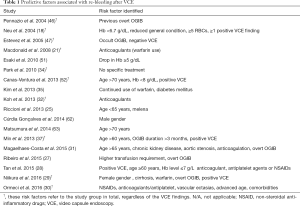
Full table
Physicians should also maintain a high level of suspicion in case of older patients since data converge to the conclusion that advanced age is a an independent factor for re-bleeding (27,28,31,52,63). This might be linked to the increased prevalence of angiodysplasia among older individuals and the high incidence of angiodysplasia when chronic kidney disease is present (2,64). Documented fall in hemoglobin below 8 g/dL as well as higher need of blood units transfusion prior to VCE mandates clinical re-evaluation and pursue of further endoscopic investigations (27,28,30,51,52).
Conclusions
More than 15 years have passed since the availability of VCE, with OGIB investigation being its predominant indication. In this setting, VCE has been established as the key element guiding subsequent invasive treatment in the subset of patients diagnosed with severe pathology. As re-bleeding risk is determined by the cardinal OGIB cause and difference regarding the prevalent lesion between Western and Eastern populations can be noted, regional variations should be also taken into consideration during follow-up. Furthermore, a negative VCE allows proper identification of those patients with low re-bleeding risk that will benefit from conservative management. This applies for the first two post-procedural years suggesting that these patients should be put under close monitoring thereafter. In all cases, presence of risk factors for re-bleeding should raise concerns about close observation.
Acknowledgements
None.
Footnote
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- ASGE Standards of Practice Committee. The role of endoscopy in the management of suspected small-bowel bleeding. Gastrointest Endosc 2017;85:22-31. [Crossref] [PubMed]
- Raju GS, Gerson L, Das A, et al. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology 2007;133:1697-717. [Crossref] [PubMed]
- Rondonotti E, Marmo R, Petracchini M, et al. The American Society for Gastrointestinal Endoscopy (ASGE) diagnostic algorithm for obscure gastrointestinal bleeding: eight burning questions from everyday clinical practice. Dig Liver Dis 2013;45:179-85. [Crossref] [PubMed]
- Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature 2000;405:417. [Crossref] [PubMed]
- Gerson LB, Fidler JL, Cave DR, et al. ACG Clinical Guideline: Diagnosis and Management of Small Bowel Bleeding. Am J Gastroenterol 2015;110:1265-87. [Crossref] [PubMed]
- Koulaouzidis A, Rondonotti E, Karargyris A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J Gastroenterol 2013;19:3726-46. [Crossref] [PubMed]
- Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol 2005;100:2407-18. [Crossref] [PubMed]
- Leighton JA, Triester SL, Sharma VK. Capsule endoscopy: a meta-analysis for use with obscure gastrointestinal bleeding and Crohn's disease. Gastrointest Endosc Clin N Am 2006;16:229-50. [Crossref] [PubMed]
- Yung DE, Koulaouzidis A, Avni T, et al. Clinical outcomes of negative small-bowel capsule endoscopy for small-bowel bleeding: a systematic review and meta-analysis. Gastrointest Endosc 2017;85:305-317.e2. [Crossref] [PubMed]
- Chen X, Ran ZH, Tong JL. A meta-analysis of the yield of capsule endoscopy compared to double-balloon enteroscopy in patients with small bowel diseases. World J Gastroenterol 2007;13:4372-8. [Crossref] [PubMed]
- Pasha SF, Leighton JA, Das A, et al. Double-balloon enteroscopy and capsule endoscopy have comparable diagnostic yield in small-bowel disease: a meta-analysis. Clin Gastroenterol Hepatol 2008;6:671-6. [Crossref] [PubMed]
- Teshima CW, Kuipers EJ, van Zanten SV, et al. Double balloon enteroscopy and capsule endoscopy for obscure gastrointestinal bleeding: an updated meta-analysis. J Gastroenterol Hepatol 2011;26:796-801. [Crossref] [PubMed]
- Van de Bruaene C, De Looze D, Hindryckx P. Small bowel capsule endoscopy: Where are we after almost 15 years of use? World J Gastrointest Endosc 2015;7:13-36. [Crossref] [PubMed]
- Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015;47:352-76. [Crossref] [PubMed]
- Cobrin GM, Pittman RH, Lewis BS. Increased diagnostic yield of small bowel tumors with capsule endoscopy. Cancer 2006;107:22-7. [Crossref] [PubMed]
- Chong AK, Chin BW, Meredith CG. Clinically significant small-bowel pathology identified by double-balloon enteroscopy but missed by capsule endoscopy. Gastrointest Endosc 2006;64:445-9. [Crossref] [PubMed]
- Delvaux M, Fassler I, Gay G. Clinical usefulness of the endoscopic video capsule as the initial intestinal investigation in patients with obscure digestive bleeding: validation of a diagnostic strategy based on the patient outcome after 12 months. Endoscopy 2004;36:1067-73. [Crossref] [PubMed]
- Neu B, Ell C, May A, et al. Capsule endoscopy versus standard tests in influencing management of obscure digestive bleeding: results from a German multicenter trial. Am J Gastroenterol 2005;100:1736-42. [Crossref] [PubMed]
- Lai LH, Wong GL, Chow DK, et al. Long-term follow-up of patients with obscure gastrointestinal bleeding after negative capsule endoscopy. Am J Gastroenterol 2006;101:1224-8. [Crossref] [PubMed]
- Redondo-Cerezo E, Gomez-Ruiz CJ, Sanchez-Manjavacas N, et al. Long-term follow-up of patients with small-bowel angiodysplasia on capsule endoscopy. Determinants of a higher clinical impact and rebleeding rate. Rev Esp Enferm Dig 2008;100:202-7. [Crossref] [PubMed]
- Macdonald J, Porter V, McNamara D. Negative capsule endoscopy in patients with obscure GI bleeding predicts low rebleeding rates. Gastrointest Endosc 2008;68:1122-7. [Crossref] [PubMed]
- Albert JG, Schulbe R, Hahn L, et al. Impact of capsule endoscopy on outcome in mid-intestinal bleeding: a multicentre cohort study in 285 patients. Eur J Gastroenterol Hepatol 2008;20:971-7. [Crossref] [PubMed]
- Viazis N, Papaxoinis K, Vlachogiannakos J, et al. Is there a role for second-look capsule endoscopy in patients with obscure GI bleeding after a nondiagnostic first test? Gastrointest Endosc 2009;69:850-6. [Crossref] [PubMed]
- Laine L, Sahota A, Shah A. Does capsule endoscopy improve outcomes in obscure gastrointestinal bleeding? Randomized trial versus dedicated small bowel radiography. Gastroenterology 2010;138:1673-1680.e1; quiz e11-2.
- Riccioni ME, Urgesi R, Cianci R, et al. Negative capsule endoscopy in patients with obscure gastrointestinal bleeding reliable: recurrence of bleeding on long-term follow-up. World J Gastroenterol 2013;19:4520-5. [Crossref] [PubMed]
- Katsinelos P, Lazaraki G, Gkagkalis A, et al. The role of capsule endoscopy in the evaluation and treatment of obscure-overt gastrointestinal bleeding during daily clinical practice: a prospective multicenter study. Scand J Gastroenterol 2014;49:862-70. [Crossref] [PubMed]
- Ribeiro I, Pinho R, Rodrigues A, et al. What is the long-term outcome of a negative capsule endoscopy in patients with obscure gastrointestinal bleeding? Rev Esp Enferm Dig 2015;107:753-8. [PubMed]
- Tan W, Ge ZZ, Gao YJ, et al. Long-term outcome in patients with obscure gastrointestinal bleeding after capsule endoscopy. J Dig Dis 2015;16:125-34. [Crossref] [PubMed]
- Niikura R, Yamada A, Nagata N, et al. New predictive model of rebleeding during follow-up of patents with obscure gastrointestinal bleeding: A multicenter cohort study. J Gastroenterol Hepatol 2016;31:752-60. [Crossref] [PubMed]
- Ormeci A, Akyuz F, Baran B, et al. What is the impact of capsule endoscopy in the long term period? World J Gastrointest Endosc 2016;8:344-8. [Crossref] [PubMed]
- Magalhães-Costa P, Bispo M, Santos S, et al. Re-bleeding events in patients with obscure gastrointestinal bleeding after negative capsule endoscopy. World J Gastrointest Endosc 2015;7:403-10. [PubMed]
- Koh SJ, Im JP, Kim JW, et al. Long-term outcome in patients with obscure gastrointestinal bleeding after negative capsule endoscopy. World J Gastroenterol 2013;19:1632-8. [Crossref] [PubMed]
- Endo H, Matsuhashi N, Inamori M, et al. Rebleeding rate after interventional therapy directed by capsule endoscopy in patients with obscure gastrointestinal bleeding. BMC Gastroenterol 2008;8:12. [Crossref] [PubMed]
- Park JJ, Cheon JH, Kim HM, et al. Negative capsule endoscopy without subsequent enteroscopy does not predict lower long-term rebleeding rates in patients with obscure GI bleeding. Gastrointest Endosc 2010;71:990-7. [Crossref] [PubMed]
- Kim JB, Ye BD, Song Y, et al. Frequency of rebleeding events in obscure gastrointestinal bleeding with negative capsule endoscopy. J Gastroenterol Hepatol 2013;28:834-40. [Crossref] [PubMed]
- Pongprasobchai S, Chitsaeng S, Tanwandee T, et al. Yield, etiologies and outcomes of capsule endoscopy in Thai patients with obscure gastrointestinal bleeding. World J Gastrointest Endosc 2013;5:122-7. [Crossref] [PubMed]
- Min YW, Kim JS, Jeon SW, et al. Long-term outcome of capsule endoscopy in obscure gastrointestinal bleeding: a nationwide analysis. Endoscopy 2014;46:59-65. [PubMed]
- Saurin JC, Delvaux M, Vahedi K, et al. Clinical impact of capsule endoscopy compared to push enteroscopy: 1-year follow-up study. Endoscopy 2005;37:318-23. [Crossref] [PubMed]
- Hindryckx P, Botelberge T, De Vos M, et al. Clinical impact of capsule endoscopy on further strategy and long-term clinical outcome in patients with obscure bleeding. Gastrointest Endosc 2008;68:98-104. [Crossref] [PubMed]
- Lorenceau-Savale C, Ben-Soussan E, Ramirez S, et al. Outcome of patients with obscure gastrointestinal bleeding after negative capsule endoscopy: results of a one-year follow-up study. Gastroenterol Clin Biol 2010;34:606-11. [Crossref] [PubMed]
- Mussetto A, Fuccio L, Dari S, et al. MiroCam capsule for obscure gastrointestinal bleeding: a prospective, single centre experience. Dig Liver Dis 2013;45:124-8. [Crossref] [PubMed]
- Xin L, Liao Z, Jiang YP, et al. Indications, detectability, positive findings, total enteroscopy, and complications of diagnostic double-balloon endoscopy: a systematic review of data over the first decade of use. Gastrointest Endosc 2011;74:563-70. [Crossref] [PubMed]
- Samaha E, Rahmi G, Landi B, et al. Long-term outcome of patients treated with double balloon enteroscopy for small bowel vascular lesions. Am J Gastroenterol 2012;107:240-6. [Crossref] [PubMed]
- Ross A, Mehdizadeh S, Tokar J, et al. Double balloon enteroscopy detects small bowel mass lesions missed by capsule endoscopy. Dig Dis Sci 2008;53:2140-3. [Crossref] [PubMed]
- Mergener K, Ponchon T, Gralnek I, et al. Literature review and recommendations for clinical application of small-bowel capsule endoscopy, based on a panel discussion by international experts. Consensus statements for small-bowel capsule endoscopy, 2006/2007. Endoscopy 2007;39:895-909. [Crossref] [PubMed]
- Pennazio M, Santucci R, Rondonotti E, et al. Outcome of patients with obscure gastrointestinal bleeding after capsule endoscopy: report of 100 consecutive cases. Gastroenterology 2004;126:643-53. [Crossref] [PubMed]
- Estévez E, González -Conde B, Vázquez -Iglesias JL, et al. Diagnostic yield and clinical outcomes after capsule endoscopy in 100 consecutive patients with obscure gastrointestinal bleeding. Eur J Gastroenterol Hepatol 2006;18:881-8. [Crossref] [PubMed]
- Apostolopoulos P, Liatsos C, Gralnek IM, et al. Evaluation of capsule endoscopy in active, mild-to-moderate, overt, obscure GI bleeding. Gastrointest Endosc 2007;66:1174-81. [Crossref] [PubMed]
- García-Compean D, Armenta JA, Marrufo C, et al. Impact of therapeutic interventions induced by capsule endoscopy on long term outcome in chronic obscure GI bleeding. Gastroenterol Clin Biol 2007;31:806-11. [Crossref] [PubMed]
- Carey EJ, Leighton JA, Heigh RI, et al. A single-center experience of 260 consecutive patients undergoing capsule endoscopy for obscure gastrointestinal bleeding. Am J Gastroenterol 2007;102:89-95. [Crossref] [PubMed]
- Esaki M, Matsumoto T, Yada S, et al. Factors associated with the clinical impact of capsule endoscopy in patients with overt obscure gastrointestinal bleeding. Dig Dis Sci 2010;55:2294-301. [Crossref] [PubMed]
- Cañas-Ventura A, Marquez L, Bessa X, et al. Outcome in obscure gastrointestinal bleeding after capsule endoscopy. World J Gastrointest Endosc 2013;5:551-8. [Crossref] [PubMed]
- Li X, Dai J, Lu H, et al. A prospective study on evaluating the diagnostic yield of video capsule endoscopy followed by directed double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Dig Dis Sci 2010;55:1704-10. [Crossref] [PubMed]
- Leighton JA, Sharma VK, Hentz JG, et al. Capsule endoscopy versus push enteroscopy for evaluation of obscure gastrointestinal bleeding with 1-year outcomes. Dig Dis Sci 2006;51:891-9. [Crossref] [PubMed]
- Prakash C, Zuckerman GR. Acute small bowel bleeding: a distinct entity with significantly different economic implications compared with GI bleeding from other locations. Gastrointest Endosc 2003;58:330-5. [PubMed]
- Somsouk M, Gralnek IM, Inadomi JM. Management of obscure occult gastrointestinal bleeding: a cost-minimization analysis. Clin Gastroenterol Hepatol 2008;6:661-70. [Crossref] [PubMed]
- Triantafyllou K, Angeletopoulou C. IMF and European co-workers attack public health in Greece. Lancet 2011;378:1459-60. [Crossref] [PubMed]
- Baumbach A, Gulis G. Impact of financial crisis on selected health outcomes in Europe. Eur J Public Health 2014;24:399-403. [Crossref] [PubMed]
- Triantafyllou K, Gkolfakis P, Viazis N, et al. A 13-year time trend analysis of 3724 small bowel video capsule endoscopies and a forecast model during the financial crisis in Greece. Eur J Gastroenterol Hepatol 2017;29:185-191. [Crossref] [PubMed]
- Marmo R, Rotondano G, Rondonotti E, et al. Capsule enteroscopy vs. other diagnostic procedures in diagnosing obscure gastrointestinal bleeding: a cost-effectiveness study. Eur J Gastroenterol Hepatol 2007;19:535-42. [Crossref] [PubMed]
- Gerson L, Kamal A. Cost-effectiveness analysis of management strategies for obscure GI bleeding. Gastrointest Endosc 2008;68:920-36. [Crossref] [PubMed]
- Cúrdia Gonçalves T, Dias de Castro F, Moreira MJ, et al. Small bowel capsule endoscopy in obscure gastrointestinal bleeding: normalcy is not reassuring. Eur J Gastroenterol Hepatol 2014;26:927-32. [Crossref] [PubMed]
- Matsumura T, Arai M, Saito K, et al. Predictive factor of re-bleeding after negative capsule endoscopy for obscure gastrointestinal bleeding: over 1-year follow-up study. Dig Endosc 2014;26:650-8. [Crossref] [PubMed]
- Holleran G, Hall B, Hussey M, et al. Small bowel angiodysplasia and novel disease associations: a cohort study. Scand J Gastroenterol 2013;48:433-8. [Crossref] [PubMed]


