External beam techniques to boost cervical cancer when brachytherapy is not an option—theories and applications
Introduction
Cervical cancers contribute significantly to the burden of cancer worldwide (~500,000 yearly incidence) (1) and in the US (~12,360 were diagnosed in 2016) (2). Ever since the first publication on afterloading low dose rate (LDR) brachytherapy (BT) in the management of gynecologic malignancies in 1960 (3), BT has been considered an integral part of the cervical cancers treatment paradigm (4).
The inclusion of BT in the treatment of cervical cancer has persistently shown to reduce local recurrence (LR) (5) and to improve overall survival (OS) compared to pelvic external beam radiotherapy (EBRT) alone (6).
Compared with other EBRT techniques, the superiority of BT is explained by its unparalleled dose distribution, characterized by low integral dose and sharp dose gradient which permits maximum sparing of the surrounding normal tissue while delivering high doses to tumor. Moreover, the radioactive sources are loaded within applicators inserted in the target volume which eliminates the requirement of additional margins to account for set up error and/or to adapt to changes in bladder and rectal filling; both represent challenges to EBRT precision.
Over the last decade, the unrivaled physical properties of brachytherapy witnessed major advances through the adoption of image guidance fortified by the incorporation of magnetic resonance (MR) multimodality imaging, optimized volumetric planning and the increased use of high dose rate (HDR) BT delivery; all of which were associated with significant improvement of treatment outcomes (7,8). Several studies confirmed the equivalence between the current HDR dose fractionation schedules and LDR BT, both in term of tumor control and toxicity (9,10). In spite of the beneficial radiobiologic characteristics of LDR, its use in cervical cancer is decreasing rapidly being replaced by HDR (11) due to practical advantages such as (12):
- Increased of flexibility of dose distribution dose through optimization of dwell position and dwell time;
- more consistent and reproducible set up due to shortened delivery time;
- enables outpatient delivery with reduction of patient discomfort and complications arising from prolonged bed rest.
However, the superiority of BT techniques is not free of limitations. This operator dependent technique requires specific skills that, if absent, can significantly affect outcomes. For instance, inadequate placement and/or displacement of ovoids reduced both local control (LC) and disease-free survival (DFS) rates, while inadequate packing reduced DFS (13). Even after adequate placement of applicator, significant inter- and intra-fraction variations can occur (14). Furthermore, there are additional aspects to the BT procedural requirements regardless of the skill of the clinician. Firstly, cervical dilation is occasionally challenging which necessitates full anesthesia with its associated operating and recovery room time increasing BT overall cost. Secondly, BT might be associated with non-trivial side effects (15) including uterine perforation, vaginal laceration and anesthesia-associated risks. Thirdly, physical constraints due to variations in vaginal accommodation, such as normal variations with age, prior pelvic procedures, or uterine malformations and/or insufficient reduction of the tumor volume preclude some patients from having adequate applicators insertion (16). Fourthly, some patients refuse BT applicator insertion, presumably due to anxiety or discomfort (16). Finally, implementing high quality volumetric image guided BT—associated with the best treatment outcomes—in routine practice was limited to only 25% of the clinics in a recent survey. Highlighting its procedural and logistical requirements (17) is not globally available.
Over the last decade, very few studies evaluated the role of EBRT as a substitute to the BT technique. Limited by old EBRT techniques delivering suboptimal dose in small population studies, the results were disappointing (6,18). Often, these studies treated patients with extensive disease where intracavitary and/or interstitial brachytherapy were not an option and where SBRT/IMRT boost was used as the last resort after significant delay. Not accounting for these crucial factors may inaccurately reflect the inferiority of a viable treatment option as reported in some population-based studies (19). Accordingly, the existing data evaluating EBRT techniques were limited to those patients who cannot undergo BT for medical or technical reasons obviously expected to have an unfavorable outcome regardless of the technique and these experiences halted any effort to re-explore EBRT as a potential contingency substitute for BT Even when BT delivery unit and/or expertise are not available, international guidelines offer EBRT boost as a weak recommendation (20).
To allow for some tumor reduction, BT is typically attempted during the last weeks of chemoradiotherapy while still not exceeding 8 weeks overall treatment time to avoid tumor repopulation. Other times, the caring team may face patient’s refusal, large cervical tumor with high lateral parametrial extension not accessible to interstitial implant and/or medical contraindications which precludes BT delivery altogether. These challenging scenarios need a formalized alternative plan executed without any further delay to deliver the intended therapeutic dose without submitting to the dogma that BT is thus irreplaceable.
We hereby propose a potential BT alternative to be rigorously tested in clinical trial for possible future execution whenever situations challenging BT feasibility arise. For the purpose of this article, HDR BT delivery and its fractionation schedule will be adopted for all comparisons due to its wide prevalence in current practice. In addition, SBRT will be distinguished from IMRT by being the technique delivering high dose per fraction (exceeding 5 Gy) since both (IMRT and SBRT) can be delivered using the same platform. For radiobiologic model comparisons, two EBRT fractionations schedules will be tested depending on the timing of their integration relative to the concurrent chemoradiotherapy schedule. If brachytherapy is deemed inadequate before starting chemoradiotherapy, IMRT with simultaneous integrated boost (SIB) boost will be the suggested technique in that scenario. Conversely, SBRT delivering high dose per fraction (4–7 fractions on alternate days) will be the preferred route in cases when BT is deemed not applicable towards the end of the conventionally fractionated chemoradiotherapy regimen.
Radiobiologic considerations
Which dose fractionations to use?
The therapeutic ratio is a fine line separating tumor control probability (TCP) from normal tissue complication probability (NTCP). This line relies on complex interactions of competing radiobiologic phenomena. Many considerations are heavily weighted to detect the optimum fractionation schedule maximizing the therapeutic ratio (21):
- Balancing overall treatment time to allow early reacting tissue regeneration while preventing accelerated tumor repopulation;
- accounting for late reacting tissue repair kinetics with judicious choice of inter-fraction interval;
- anticipating accentuation of hypoxic radioresistance with increasing dose per fraction while considering the tumor and normal tissue alpha/beta (α/β) ratios balance.
First model
Insufficient interfraction repair concern
In the first model, we will discuss the SBRT technique employed towards the end of the chemoradiotherapy schedule should situations arise and prevent HDR BT application at this later stage of the treatment package. Endorsed by the American Brachytherapy Society, 30 Gy in 5 fractions was the most common HDR fractionation schedule over the past decade (22). For patients receiving SBRT, a similar fractionation scheme was proposed; therefore, negligible radiobiologic differences could be expected when 30 Gy in 5 fractions is delivered with either HDR BT or SBRT (setting aside the dose distribution variation with each technique that is clearly advantageously hotter in brachytherapy). Comparable to more protracted regimens (as in LDR), the hypofractionated schedules (adopted in HDR BT or SBRT) maybe as forgiving regarding the sparing of normal tissue refuting initial claims of lower rate of interfraction repair of sublethal damage at high dose per fraction (>5 Gy) (23). Indeed, full repair is expected when appropriate interfraction interval is allowed thus accounting for the late-reacting normal tissue repair half time averaging about 2.5 hours (24).
Overall treatment time concern
These hypofractionated regimens are theoretically more advantageous in slowly proliferating tumor with low α/β ratio (21), however, the aim of hypofractionation in the cervical cancer setting is to target rapid cervical cancer cell repopulation through avoiding overall treatment time prolongation (21); a notorious parameter that contributes to detrimental treatment outcome (25). This feature is essentially important in cervical tumors which are characterized by short doubling time (26), accelerated repopulation and high α/β ratio (27).
Other lingering questions
Although this fractionation was proven to be as clinically efficient as the traditional LDR schedules (9,10), few issues require further characterization: First, the optimum schedule taking in account late reacting normal low α/β ratio to provide extra sparing remains to be defined. Second, it is required to address the concern on accelerated tumor repopulation occurring during the 2 to 5 days intervals typically allowed between high dose fraction [to avoid excessive toxicity if interdigitating HDR BT or SBRT within EBRT course is not possible (28)]. Third, the integration of pharmaceuticals with this hypofractionated radiotherapy and their full extent of radiosensitization need further depiction.
Second model
Potential shortening of the overall treatment time
In the second model, we assess the feasibility of adopting IMRT with cervical SIB should condition preventing BT utilization are decided before initiation of the chemoradiotherapy treatment. In contrast to the previous model, the IMRT paradigm provides a fractionation scheme integrating the boost dose within the 5–6 weeks course to deliver the total dose in a shorter duration without employing large fraction size; thus, potentially improving the therapeutic ratio. Guerrero et al. proposed simultaneous integrated IMRT boost that, in only 25 fractions, deliver 77.5 Gy (in 3.1 Gy per fraction) to the cervical planning target volume (PTV) and 45 Gy (in 1.8 Gy per fraction) to the pelvic PTV. In their equivalent uniform dose (EUD) adjusted model, the calculated biologically effective dose (BED) for normal tissue, while delivering the same BED for the tumor, was 154.2 vs. 162 Gy3 for IMRT vs. HDR boost, respectively; clearly favoring the former (29). Although this study assessed the dose volume histogram derived from IMRT plans compared to point A HDR BT plans, the concept of reducing the treatment duration to 5 weeks overcoming any accelerated repopulation occurring with longer regimen may potentially obviate the need of utilizing high dose per fraction. Incorporating adaptive replanning strategy may further accentuate the precision by allowing modifications of the target volumes with the anticipated tumor shrinkage.
Dose painting specifically targeting hypoxic radioresistant foci
Moreover, the IMRT model may be hypothetically preferred in the presence of hypoxia. Within the cervical tumor notoriously associated with radioresistance and poor treatment outcome. The percentage of hypoxic cells within individual cervical tumors is in the 20–60% range (30). While BT characteristic dose distribution delivering extreme high doses within the tumor (at the vicinity of the radioactive source) and overcoming the radioresistance of the hypoxic clones, a more protracted schedule—with lower oxygen enhancement ratio (OER)—may relatively be more efficient in reducing chronic hypoxia through reoxygenation occurring with tumor shrinking (31). Moreover, a comparable dose distribution can be generated with optimized SBRT or IMRT plans to create subvolumes receiving comparable high doses. These optimized EBRT plans can even surpass BT dose distribution by specifically targeting areas of hypoxia employing multimodality imaging such as PET (32) and MRI (33).
Simulation models
The biologic efficacy of the short integrated IMRT fractionation schedule (SIF) proposed by Guerrero et al. (29) versus the conventional schedule (CF) consisting of 5 weeks of EBRT interdigitating with a hypofractionated boost via SBRT (or HDR BT) is compared using the subsequent equations.
Various BED estimates were calculated using the linear quadratic formalism and its modifications (34) where an α/β of 3 and 10 Gy reflected late-responding tissue and tumor,respectively (Eq.[1]).
BED = nd [1+ d/(α/β)] [1]
Where n is the number of fractions and d is the dose per fraction.
Taking repopulation and overall treatment time in consideration, the formula is modified as follows:
BED = nd [1+ d/(α/β)] − TF [2]
TF = 0.693 (TD − TK)/α Tpot [3]
TD is the total treatment duration; 35 and 56 days in the IMRT (delivered in 25 fractions over 5 weeks) and SBRT/HDR-BRT (delivered in 5 fractions interdigitating with EBRT and for additional 3 weeks after EBRT) models, respectively. TK is the time where accelerated repopulation kicks in ranging from 21 to 35 days (35), Tpot is the potential tumor doubling time ranging from 3 to 6 days (36) and α reflecting tumor radiosensitivity ranging from 0.1 Gy-1 (radioresistant) to 0.5 Gy-1 (radiosensitive) (36).
The total BED is calculated using the following equation:
BEDtotal = BED EBRT + BED BT − TF [4]
As hypoxic foci constitutes 20–60% of individual tumors (30) and consequently negatively impact tumor radiosensitivity and disease control (37), adaptation of linear quadratic formula (38) was attempted using the following:
BEDhypoxic= nd [(1/qα) + d/(qβ2.α/β)]
−0.693 (TD−TK)/α Tpot [5]
In this equation, qα and qβ are the OER at low and high dose per fractions, respectively. Values of qα and qβ were set at 1.5 and 3, respectively (39). As OER increases with hypofractionation, the values of qα and qβ were increased by 10% in the SBRT/HDR dose fractionation model (39).
Considering enough reoxygenation occurring in the first 1–3 weeks of radiotherapy due to tumor shrinkage, the hypoxic component (H) will be set to 0.2, which brings the cumulative BED equation to:
BEDcumulative = H (BEDhypoxic) + (1−H) (BEDtotal) [6]
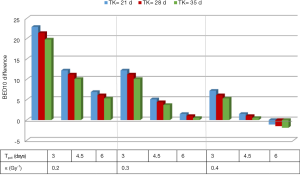
From a tumoricidal perspective, a higher total dose delivering a higher BED10 would potentially results in higher TCP. As shown in Figure 1 and Table 1, the estimated BED10 was higher in the SIF in most scenarios (with different ranges of Tk, Tpot and α). The BED10 difference between the SIF and CF reaches up to 22.9 Gy in radioresistant tumor (α =0.2 Gy-1) with rapid Tpot, and fast Tk. This difference decreases with increasing Tk, Tpot and α. Indeed, BED10 estimation favored CF only in radiosensitive tumor with high α (0.4 Gy-1). In this uncommon scenario, the BED10 difference was only 1.9 Gy. To examine this BED10 difference as a function of tumor volume, the TCP, plotted against tumor volume, favored SIF and increased with larger tumor size as shown in Figure 2. In this simulation model, the tumor volume ranged from 10–200 cm3 (40) and the tumor cell density was 108 in the TCP equation (38) that included 0.3 Gy-1, 28 and 4.5 days for α, TK and Tpot respectively.

Full table
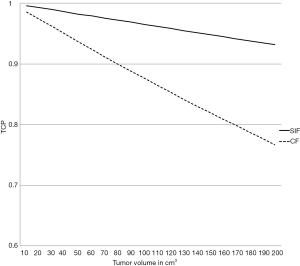
TCP = exp [−C. V. exp (−α. BED10)] [7]
Where C is clonogens density per cm3 and V is the tumor volume in cm3.
Regarding sparing of late reacting tissue, the estimated BED3 favored SIF over CF where the estimates were 155 and 162 Gy, respectively (assuming a similar Dmax and D2cc).
In the situations where BT feasibility won’t be applicable, a well-executed IMRT plan addressing target volume motions and dose distribution issues, the proposed CIF may provide an equally effective alternative, especially in radioresistant hypoxic tumor with shorter time to repopulation and shorter doubling time.
Technical considerations for appropriate application of EBRT techniques in case of BT non-feasibility
Dosimetric perspective
In absence of the BT option, SBRT is capable of emulating BT characteristic dose distribution through exploiting multiple non-coplanar beams intersecting at the target volume to deliver a high inhomogeneous dose to the tumor while shaping dose away from surrounding normal organs. Simultaneously, SBRT precise targeting allows dose escalation to the cervix to a dose fractionation schedule comparable to that delivered by HDR BT.
Newer techniques may offer dose distribution not exceedingly inferior to that provided by BT
A number of dosimetric studies advocated superior target coverage and sparing of organs at risk in favor of SBRT (41-43). For instance, Cengiz et al. generated SBRT plans for 11 stage IIB cervical cancer patients and compared the dose distributions with those delivered using BT. The rectal dose to 1 cc (d1cc) was (5.09 vs. 6.05 Gy, P=0.02), the bladder d1cc (6.78 vs. 8.76 Gy, P=0.04) and the median target coverage by the 100% isodose line was (99.1% vs. 50.7%, P<0.05); all favoring SBRT plans (the study however was limited by BT dose prescription to point A) (41). Similarly, the volumetric modulated arc therapy (VMAT) dosimetric plans [generated for 51 gynecologic cancer patients receiving the initial 45–50.4 Gy via three dimensional conformal radiotherapy (3DCRT) and a boost of 6 Gy for 3–4 fractions via BT] demonstrated that SBRT-compared to BT- is capable of significantly reducing rectal d1cc, d2cc and maximum dose (dmax) with comparable bladder and bowel dose; however, the integral dose and the PTV coverage favored BT (44). These results are discordant with Georg et al. dosimetric study in which image guided BT was superior to EBRT plans using either photon or proton radiotherapy. For the modulated photon plans, the target coverage was compromised if optimization did not exceed the d1cc, d2cc achieved by BT; however, the modulated proton plans—optimized to target volume with 3 mm PTV expansion—were comparable to BT in term of PTV dose coverage (45).
In contrast to SBRT, the premise of IMRT is the manipulation of numerous tiny subdivisions of beams resulting in nonuniform beam intensity to allow a highly conformal overall treatment field (46). Given that each beam can be altered individually, fine control of dose distribution is possible, a desirable characteristic permitting dose painting (i.e., SIB). For example, the larger pelvic PTV can be treated to a low dose per fractions (e.g., 1.8 Gy); while simultaneously, the cervical PTV boost volume radiation can receive a higher daily dose (e.g., 3.1 Gy) and thus, within 25 fractions, the total dose to the pelvic and the cervical target volumes are 45 and 77.5 Gy, respectively. As discussed previously, the integrated boost concept is attractive from a radiobiologic standpoint in a rapidly proliferating tumor where reduction of overall treatment time maybe beneficial (29). This concept was evaluated in 20 stage III cervical cancer patients yielding improved LC trend (47). In contrast to SBRT and BT, IMRT is employed to shape dose distribution for larger target volumes to deliver a more protracted and homogeneous dose fractionation schedule (>5 fractions). Dosimetric studies comparing IMRT and BT in this setting are lacking and most of the studies addressed IMRT superiority compared with other EBRT techniques rather than compared with BT. For instance, IMRT was shown to reduce grade 2 and eliminate grade 3 or higher bowel toxicity when compared to conformal whole pelvic radiotherapy with possible reduction of chemoradiotherapy associated bone marrow toxicity (48). In patients unable to undergo brachytherapy after completing 45–50 Gy, additional 20–30 Gy delivered via IMRT was superior to conformal radiotherapy in terms of rectal/bladder sparing as well as target coverage (49). That said, the dosimetric superiority of BT remains unrivaled by any EBRT techniques and only shortening of the overall treatment time provided by IMRT SIB plan may potentially offset some of its dose distribution short coming; Thus, justifying its consideration whenever BT is not an option.
Accounting for target motion: an ongoing dilemma
The continuous non-systemic cervical target volume motion precipitated by variable bladder and rectal filling can be as large as 18 mm (50). During the treatment course, the cervical tumor undergoes significant volume shrinkage (ranging from 50% to 79%) (51); however, these volume reductions cannot offset the need for PTV margins to account for the magnitude of target motion. For example, the mean reduction in cervical volume was 62.3%; yet, a large PTV margin was still necessary as the mean maximum changes in cervical volume perimeter may respectively reach up to 2.3, 1.3, 1.7, 1.8, 0.76 and 0.94 cm in superior, inferior, anterior, posterior, right and left directions (52). In order to account for this large motion uncertainty, continuous tracking and/or immobilization of the target volume is imperative for precise EBRT delivery.
In an attempt to increase reproducibility, Pedicini et al. reported on applicator-guided volumetric arc therapy. In their experience, the CT compatible vaginal cylinder, through providing spatial registration and immobilization of the gynecologic organs, minimized target volume motion variability allowing 2 mm PTV margin. By reducing cervical target volume motion, this applicator allowed lower rectal dose when compared with BT (44).
In addition to immobilization, tracking cervical motion using gold fiducial markers—a surrogate for cervical tumor motion—to follow systemic and random target motion was advocated as another alternative to enhance SBRT delivery. This technique was employed in 10 cervical cancer patients demonstrating that the PTV margin can be safely reduced to 6.9, 6.7, and 8.3 mm to allow for precise conformal boost delivery. Tighter margins can even be applied with real time tracking (53). While, immobilization or tracking methods provide a mean to reduce cervical clinical target volume (CTV) to PTV margins, these solution need to be integrated within pelvic lymph nodes CTV to PTV margin expansion if EBRT boost is delivered simultaneously.
Lessons learned from other tumors to improve the EBRT delivery precision
The efficiency of emulating HDR BT dose distribution using SBRT (termed virtual HDR) is currently under investigation in another pelvic organ—the prostate—with promising preliminary results (54) and the literature documenting the feasibility of SBRT and its effectiveness in this setting is expanding (55,56). Delivering accurate highly conformal EBRT dose is challenged by the moving prostate target volume that, akin to the cervical CTV motion, is mostly due to unpredictable bladder/rectal filling (50). Nevertheless, the precision of SBRT tracking strategies using fiducial markers permitted the reduction of PTV margin with increased reproducibility (57). A rectal balloon to minimize prostate motion was equally effective (58), thus suggesting the possibility of using similar strategies in cervical radiotherapy. Likewise, the non-invasive ultrasound online tracking was successfully used in prostate radiotherapy (59), and recently, proven to be efficient in cervical cancer tracking for daily IMRT (60).
Unlike prostate cancer, cervical tumors undergo substantial shrinkage during the treatment course (52) displaying similar patterns to those observed head and neck squamous cell carcinoma (61) and justifying the need for adaptive replanning to minimize radiation dose to normal tissue while maintaining adequate coverage to target volume (62). Adaptive strategies were proposed in cervical cancer radiotherapy (63) and, with the current soft image registration software (64); frequent plan modification to the shrinking tumor volume can permit more sparing of normal tissue.
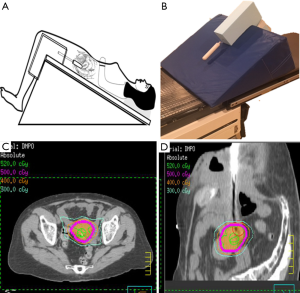
In our limited series of patients unable to undergo brachytherapy, we employed SBRT with good initial (unpublished) results (OM). Using a reverse Trendelenburg position as shown in Figure 3A, the sigmoid and small intestinal loops located near the cervical target volume are displaced superiorly. A vaginal cylinder and/or gold fiducial marker are utilized to track the target volume and minimize the deformability of the vagina(Figure 3B). This set up permitted reduction of the PTV expansion to 3 mm. For treatment planning, dose constraints akin to those used in brachytherapy (5-fraction boost) were adopted leading to achieving target volume D90 of 5–5.5 Gy per fraction while limiting the bladder, rectum and sigmoid d2cc to 70–80% of the prescription dose (Figure 3C,D). The cumulative equivalent dose to target volume ranged between 75–82 Gy.
Clinical evidence supporting brachytherapy alternatives
PubMed was used to search the literature systematically for studies published in English between January 1st 1990 and July 7th 2015. Search terms included “Cervical Cancers” or “Gynecologic cancer” with “Brachytherapy Alternatives”, “radiosurgery”, “Stereotactic body radiotherapy”, “SBRT”, “Intensity Modulated Radiation Therapy boost”, “Simultaneous Integrated Boost” and “IMRT Boost”. The abstracts of these articles were screened by two physicians (SK and OM) to assess their eligibility for the purpose of the review and yielded 78 publications. The search was restricted to studies reporting outcomes on five or more patients whose primary cervical disease was boosted with EBRT instead of BT with at least 4 months of follow-up. Only nine studies fulfilled these criteria and were included in the analysis. The remaining studies were excluded either for not reporting on clinical outcomes (the primary focus of these studies was dosimetric or adaptive replanning strategies), for only addressing review or database query, for employing a combination of IMRT and BT, for targeting the pelvic and/or paraaortic target volumes without replacing BT or for managing recurrent disease using SBRT or IMRT.
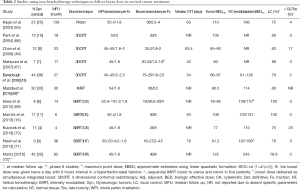
As shown in Table 2, limited number of studies evaluated high-tech EBRT as legitimate BT alternative. The studies were retrospective, heterogeneous in dose, fractionation and technique along with various follow up time and small population size. Furthermore, some studies were not restricted to cervical cancer including other gynecologic tumors. Documentation of dosimetric parameters for normal organs was not uniform which prevented against establishing a correlation between dose and toxicity. Yet, most studies delivered 45–50.4 Gy in 1.8–2 Gy per fraction to the pelvic PTV.
Full table
High-tech EBRT boost in the definitive management of cervical cancer patients was mostly attempted when BT delivery was not possible for anatomical reason or for patient refusal. In these studies, the boost dose was delivered sequentially after pelvic irradiation and it ranged 16–36 Gy in 1.8–6 Gy per fraction. Matsuura et al. study was the exception. A hyperfractionated schedule was adopted in this study where a smaller conformal boost volume (1.2–1.6 Gy per fraction) was started in the 4th week concomitant with pelvic irradiation and continued after the 5th week with twice a day fractionation (minimum of 6 hours interval between fractions). The 2-year LC was 85.7% with only 2 out of 7 patients reporting grade 2 rectal bleeding as the highest toxicity. In this study no specific image guidance radiotherapy (IGRT) was used and the CTV was uniformly expanded by 0.5–1 cm to generate the PTV (67).
Conformal RT technique was similarly employed in Barraclough et al. (18), Chan et al. (49) and Park et al. studies (66) which reported 79%, 83% and 60% 2-year LC, respectively. No grade 3 or higher late toxicity was observed in a later study that utilized real time tracking of three gold fiducial markers (implanted in the cervix) to deliver a higher total and a higher dose per fraction (30 and 5 Gy per fraction). Perhaps, the absence of IGRT can explain the higher toxicity rate observed in Barraclough et al. and Chan et al. studies (2% and 17% late grade 3 urinary and late grade 3 rectal toxicity were reported, respectively) despite delivering a lower total dose with lower BED. On the other hand, SBRT was the technique of choice in all the four recent studies that delivered 16–30 Gy in 2–6 Gy per fraction to the cervical target volume not amenable for BT. The preliminary results of three studies were promising (LC rates ranging from 78–100% and negligible late toxicity) although the median follow up duration was relatively short (6–36 months) (16,69,71). The study by Kubicek et al. was the exception with a paradoxically high late rectal toxicity (25% grade 3 or higher) in spite of using MR imaging to define the CTV, 0.5 cm CTV to PTV expansion, Cyberknife (CK) (Accuray Incorporated, Sunnyvale, CA, USA) to track cervical implanted gold fiducial for increased SBRT precision. In their heterogeneous patient population with short median follow-up, they included only four cervical squamous cell carcinoma patients treated for primary stage IIIB disease where 2 patients received 25 Gy in 5 fractions, 1 patient received 15 Gy in 3 fractions (in addition to 12 Gy HDR BT) and 1 patient received 5 Gy in single fraction before developing a stroke and going to hospice. Only the patient who could not complete the treatment developed recurrence while the remaining three patients remained disease-free (70). The CK platform tracking cervical tumor with implanted markers was similarly adopted in Haas et al. and Marnitz et al. studies yielding 100% LC without any grade-3 toxicity (16,69). This high LC rate was not replicated in the study by Hsieh et al. (22% had local failure at 3 years) partially explained by the inclusion of patients with advanced cervical disease and/or prolonged overall treatment time (median 79 days). After delivering 50–54 Gy to the pelvis, Helical Tomotherapy (HT) (TomoTherapy Inc., Madison, WI, USA) was employed to obtain a megavoltage CT image before each fraction; thus ensuring precise and consistent radiotherapy delivery and potentially avoiding late severe toxicity (71).
High-tech radiotherapy delivery in this setting was not limited to photon beam. Kagei et al. reported the long term follow up (11.5 years median follow-up duration) of 25 cervical cancer patients treated with proton beam boost using passively scattered technology. Compared to historical BT series, impressive LC rates were reported (100% and 61% for stage IIB and IIIB/IVA, respectively) with a median tumor dose of 86 Gy and grade 4 gastrointestinal or genitourinary side effects were 4% (65).
Literature addressing SBRT/IMRT as a BT alternative in cervical cancer patients is limited, with wide heterogeneity of treatment parameters. As shown in Table 2, the different dose/fractionation yielded variable tumor BED10 (from 66 to 100 Gy10) and late normal tissue BED3 (from 91 to 197 Gy3) which prohibited correlating treatment outcome with either BED estimates or BED constraints established in BT literature. By mirroring BT fractionation experience while respecting dosimetric/volumetric constraints (73), an established BED (associated with a specific outcome) can guide the selection of optimum SBRT/IMRT dose/fractionation schedule. Yet, such correlation is hard to define: While Petereit et al. could not relate the BED at Point A with either pelvic control or toxicity (74), other investigators demonstrated excessive toxicity as rectal BED3 exceeded certain threshold [>125 Gy3 for rectal point (75,76) or >140 Gy3 for rectal maximum dose (77)]. Similar association between LC/toxicity and BED could not be elucidated based on the current SBRT/IMRT boost studies. Recently, two phase II studies reported promising preliminary results of EBRT boost in patients not candidate for brachytherapy. The first study by Mazzola et al. employed IMRT plans delivering 54 Gy to the pelvis concomitant with 66 Gy in 33 fractions to the cervical gross disease and they reported 80% 3-year LC rates without severe toxicity in 30 cervical cancer patients 70 years or older who were unable to undergo brachytherapy (68). The second study delivered 40 Gy in 5 fractions using SBRT technique after 45 Gy in 25 fractions to the whole pelvis. The SBRT boost was not associated with any grade 3–4 toxicity and the 5-year LC was 78.5% (72).
Globally, it appears that the treatment outcomes of properly executed high-tech EBRT boost may not be inferior to those reported in BT series (before volumetric image guided brachytherapy era). For instance, the LC in HDR BT studies ranged from 62% to 84% (10,75,78,79). And, the BT associated major complication rates can reach up to 10% (80-82) that may include fatality (1.4%) as reported by Ferrigno et al. (78). These comparable results of EBRT and BT boost can be explained by the fact that, although the superiority of the BT physical dose distribution is undeniable (45), the implementation of a perfect brachytherapy implant is not devoid of limitations with high interfraction and interpractitioner variability. Such variability should be reduced in properly executed EBRT techniques. Akin to modern BT technique, execution of EBRT boost requires applying rigorous multimodality imaging using the MRI to better define target volumes, robust plan optimization to provide accurate sparing of organs at risk, precise treatment delivery through minimizing target volume motion with applicator guided RT or through IGRT tracking techniques, use of adaptive replanning strategies that can be modified based on biologic imaging and potentially optimizing the fractionation schedules based on biomarkers reflecting tumor kinetics. If intracavitary/interstitial BT expertise is not available and/or if extrafascial hysterectomy is not possible, only in these settings when a formalized EBRT plan applying these parameters should be considered as a true contingency BT substitute. Deviation from these parameters should be avoided due to expected inferior treatment outcomes.
Cost perspectives for EBRT techniques in case of BT non-applicability
Healthcare decisions are increasingly defined by cost considerations. From the patient perspective, minimizing costs will enhance acceptance of and adherence to treatment. From a reimbursement perspective, cost-effective treatment is preferentially reimbursed by insurance companies. Whereas sharp decrease in the use of BT in gynecologic cancers in the last two decades reported by Han et al. (6) could be explained by cost pressure, EBRT techniques boosting cervical disease due to BT non-feasibility should not substantially add to the cost concern.
We assessed payer costs of comparable BT, IMRT, and SBRT treatment plans using Medicare 2014 data (83). The radiotherapy charges were summed and compared for representative plans for these three treatment options (Table 3). Since chemotherapy and additional imaging (MRI) will be equally adopted for each of these modalities, their costs were not included. Moreover, we did not include the costs of operating and recovery room time occasionally needed for cervical dilation and sleeve placement for simplicity and for exclusively describing radiotherapy costs. Even without accounting for anesthesia cost, properly executed IMRT or SBRT plans were provided at comparable rates. Formal cost effectiveness analysis, including Markov modeling, could not be performed due to lack of enough follow-up data on patient receiving EBRT boost for reliable evidence synthesis.
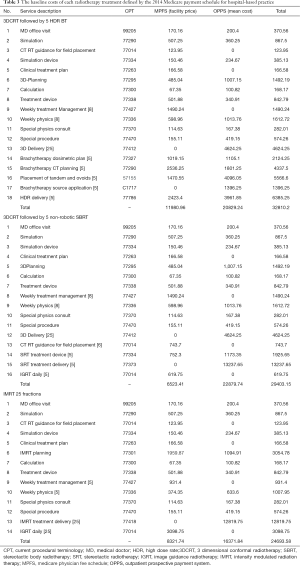
Full table
Conclusions
Brachytherapy boost to eradicate residual cervical disease after pelvic irradiation is the standard of care. Interstitial BT or extrafascial hysterectomy is the immediate approved alternatives. If for any reason these alternatives cannot be performed in a timely manner, properly designed IMRT or SBRT boost should be considered and applied. This review advocates the need of a formalized back up plan employing properly executed EBRT technique in these limited situations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cancer IAfRo. Cervical cancer estimated incidence, mortality and prevalence worldwide in 2012. World Health Organization. Available online: http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Henschke UK. “Afterloading” applicator for radiation therapy of carcinoma of the uterus. Radiology 1960;74:834. [Crossref] [PubMed]
- Viswanathan AN, Thomadsen B. American Brachytherapy Society Cervical Cancer Recommendations C, American Brachytherapy S. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles. Brachytherapy 2012;11:33-46. [Crossref] [PubMed]
- Hanks GE, Herring DF, Kramer S. Patterns of care outcome studies. Results of the national practice in cancer of the cervix. Cancer 1983;51:959-67. [Crossref] [PubMed]
- Han K, Milosevic M, Fyles A, et al. Trends in the utilization of brachytherapy in cervical cancer in the United States. Int J Radiat Oncol Biol Phys 2013;87:111-9. [Crossref] [PubMed]
- Potter R, Georg P, Dimopoulos JC, et al. Clinical outcome of protocol based image (MRI) guided adaptive brachytherapy combined with 3D conformal radiotherapy with or without chemotherapy in patients with locally advanced cervical cancer. Radiother Oncol 2011;100:116-23. [Crossref] [PubMed]
- Lindegaard JC, Fokdal LU, Nielsen SK, et al. MRI-guided adaptive radiotherapy in locally advanced cervical cancer from a Nordic perspective. Acta Oncol 2013;52:1510-9. [Crossref] [PubMed]
- Lertsanguansinchai P, Lertbutsayanukul C, Shotelersuk K, et al. Phase III randomized trial comparing LDR and HDR brachytherapy in treatment of cervical carcinoma. Int J Radiat Oncol Biol Phys 2004;59:1424-31. [Crossref] [PubMed]
- Patel FD, Sharma SC, Negi PS, et al. Low dose rate vs. high dose rate brachytherapy in the treatment of carcinoma of the uterine cervix: a clinical trial. Int J Radiat Oncol Biol Phys 1994;28:335-41. [Crossref] [PubMed]
- Grover S, Harkenrider MM, Cho LP, et al. Image Guided Cervical Brachytherapy: 2014 Survey of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys 2016;94:598-604. [Crossref] [PubMed]
- Conformal Therapy: Treatment Planning, Treatment Delivery, and Clinical Results. In: Gunderson L, Tepper J. editors. Clinical radiation oncology. Third edition. 2012.
- Viswanathan AN, Moughan J, Small W Jr, et al. The quality of cervical cancer brachytherapy implantation and the impact on local recurrence and disease-free survival in radiation therapy oncology group prospective trials 0116 and 0128. Int J Gynecol Cancer 2012;22:123-31. [Crossref] [PubMed]
- Nesvacil N, Tanderup K, Hellebust TP, et al. A multicentre comparison of the dosimetric impact of inter- and intra-fractional anatomical variations in fractionated cervix cancer brachytherapy. Radiotherapy and oncology. Radiother Oncol 2013;107:20-5. [Crossref] [PubMed]
- Kirchheiner K, Nout RA, Czajka-Pepl A, et al. Health related quality of life and patient reported symptoms before and during definitive radio(chemo)therapy using image-guided adaptive brachytherapy for locally advanced cervical cancer and early recovery - a mono-institutional prospective study. Gynecologic oncology 2015;136:415-23. [Crossref] [PubMed]
- Marnitz S, Kohler C, Budach V, et al. Brachytherapy-emulating robotic radiosurgery in patients with cervical carcinoma. Radiation oncology 2013;8:109. [Crossref] [PubMed]
- Viswanathan AN, Creutzberg CL, Craighead P, et al. International brachytherapy practice patterns: a survey of the Gynecologic Cancer Intergroup (GCIG). Int J Radiat Oncol Biol Phys 2012;82:250-5. [Crossref] [PubMed]
- Barraclough LH, Swindell R, Livsey JE, et al. External beam boost for cancer of the cervix uteri when intracavitary therapy cannot be performed. Int J Radiat Oncol Biol Phys 2008;71:772-8. [Crossref] [PubMed]
- Gill BS, Lin JF, Krivak TC, et al. National Cancer Data Base analysis of radiation therapy consolidation modality for cervical cancer: the impact of new technological advancements. Int J Radiat Oncol Biol Phys 2014;90:1083-90. [Crossref] [PubMed]
- Chuang LT, Temin S, Berek JS. Management and Care of Women With Invasive Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Clinical Practice Guideline Summary. J Oncol Pract 2016;12:693-6. [Crossref] [PubMed]
- Fowler JF. Biological factors influencing optimum fractionation in radiation therapy. Acta oncologica 2001;40:712-7. [Crossref] [PubMed]
- Viswanathan AN, Beriwal S, De Los Santos JF, et al. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: high-dose-rate brachytherapy. Brachytherapy 2012;11:47-52. [Crossref] [PubMed]
- Brenner DJ, Hall EJ. Fractionated high dose rate versus low dose rate regimens for intracavitary brachytherapy of the cervix. I. General considerations based on radiobiology. Br J Radiol 1991;64:133-41. [Crossref] [PubMed]
- Orton CG. High-dose-rate brachytherapy may be radiobiologically superior to low-dose rate due to slow repair of late-responding normal tissue cells. Int J Radiat Oncol Biol Phys 2001;49:183-9. [Crossref] [PubMed]
- Chen SW, Liang JA, Yang SN, et al. The adverse effect of treatment prolongation in cervical cancer by high-dose-rate intracavitary brachytherapy. Radiother Oncol 2003;67:69-76. [Crossref] [PubMed]
- Tsang RW, Fyles AW, Kirkbride P, et al. Proliferation measurements with flow cytometry Tpot in cancer of the uterine cervix: correlation between two laboratories and preliminary clinical results. Int J Radiat Oncol Biol Phys 1995;32:1319-29. [Crossref] [PubMed]
- Thames HD, Bentzen SM, Turesson I, et al. Time-dose factors in radiotherapy: a review of the human data. Radiother Oncol 1990;19:219-35. [Crossref] [PubMed]
- King CR, Brooks JD, Gill H, et al. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 2009;73:1043-8. [Crossref] [PubMed]
- Guerrero M, Li XA, Ma L, et al. Simultaneous integrated intensity-modulated radiotherapy boost for locally advanced gynecological cancer: radiobiological and dosimetric considerations. Int J Radiat Oncol Biol Phys 2005;62:933-9. [Crossref] [PubMed]
- Fyles A, Milosevic M, Hedley D, et al. Tumor hypoxia has independent predictor impact only in patients with node-negative cervix cancer. J Clin Oncol 2002;20:680-7. [Crossref] [PubMed]
- Lee SW, Suh CO, Chung EJ, et al. Dose optimization of fractionated external radiation and high-dose-rate intracavitary brachytherapy for FIGO stage IB uterine cervical carcinoma. Int J Radiat Oncol Biol Phys 2002;52:1338-44. [Crossref] [PubMed]
- Dehdashti F, Grigsby PW, Mintun MA, et al. Assessing tumor hypoxia in cervical cancer by positron emission tomography with 60Cu-ATSM: relationship to therapeutic response-a preliminary report. Int J Radiat Oncol Biol Phys 2003;55:1233-8. [Crossref] [PubMed]
- Hallac RR, Ding Y, Yuan Q, et al. Oxygenation in cervical cancer and normal uterine cervix assessed using blood oxygenation level-dependent (BOLD) MRI at 3T. NMR in biomedicine 2012;25:1321-30. [Crossref] [PubMed]
- Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol 1989;62:679-94. [Crossref] [PubMed]
- Buffa FM, Davidson SE, Hunter RD, et al. Incorporating biologic measurements (SF(2), CFE) into a tumor control probability model increases their prognostic significance: a study in cervical carcinoma treated with radiation therapy. Int J Radiat Oncol Biol Phys 2001;50:1113-22. [Crossref] [PubMed]
- Gasinska A, Fowler JF, Lind BK, et al. Influence of overall treatment time and radiobiological parameters on biologically effective doses in cervical cancer patients treated with radiation therapy alone. Acta oncologica 2004;43:657-66. [Crossref] [PubMed]
- Tatum JL, Kelloff GJ, Gillies RJ, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 2006;82:699-757. [Crossref] [PubMed]
- Jones B, Dale RG. Mathematical models of tumour and normal tissue response. Acta oncologica 1999;38:883-93. [Crossref] [PubMed]
- Wenzl T, Wilkens JJ. Theoretical analysis of the dose dependence of the oxygen enhancement ratio and its relevance for clinical applications. Radiat Oncol 2011;6:171. [Crossref] [PubMed]
- Lim K, Chan P, Dinniwell R, et al. Cervical cancer regression measured using weekly magnetic resonance imaging during fractionated radiotherapy: radiobiologic modeling and correlation with tumor hypoxia. Int J Radiat Oncol Biol Phys 2008;70:126-33. [Crossref] [PubMed]
- Cengiz M, Dogan A, Ozyigit G, et al. Comparison of intracavitary brachytherapy and stereotactic body radiotherapy dose distribution for cervical cancer. Brachytherapy 2012;11:125-9. [Crossref] [PubMed]
- Sethi RA, Jozsef G, Grew D, et al. Is there a role for an external beam boost in cervical cancer radiotherapy? Front Oncol 2013;3:3. [Crossref] [PubMed]
- Neumann O, Kluge A, Lyubina O, et al. Robotic radiosurgery as an alternative to brachytherapy for cervical cancer patients. Strahlentherapie und Onkologie: Strahlenther Onkol 2014;190:538-45. [Crossref] [PubMed]
- Pedicini P, Strigari L, Caivano R, et al. Local tumor control probability to evaluate an applicator-guided volumetric-modulated arc therapy solution as alternative of 3D brachytherapy for the treatment of the vaginal vault in patients affected by gynecological cancer. J Appl Clin Med Phys 2013;14:4075. [Crossref] [PubMed]
- Georg D, Kirisits C, Hillbrand M, et al. Image-guided radiotherapy for cervix cancer: high-tech external beam therapy versus high-tech brachytherapy. Int J Radiat Oncol Biol Phys 2008;71:1272-8. [Crossref] [PubMed]
- Halperin EC, Perez CA, Brady LW. Perez and Brady’s principles and practice of radiation oncology. 5th edition. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008: xxxii, 2106.
- Kavanagh BD, Gieschen HL, Schmidt-Ullrich RK, et al. A pilot study of concomitant boost accelerated superfractionated radiotherapy for stage III cancer of the uterine cervix. Int J Radiat Oncol Biol Phys 1997;38:561-8. [Crossref] [PubMed]
- Mell LK, Kochanski JD, Roeske JC, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:1356-65. [Crossref] [PubMed]
- Chan P, Yeo I, Perkins G, et al. Dosimetric comparison of intensity-modulated, conformal, and four-field pelvic radiotherapy boost plans for gynecologic cancer: a retrospective planning study. Radiat Oncol 2006;1:13. [Crossref] [PubMed]
- Haripotepornkul NH, Nath SK, Scanderbeg D, et al. Evaluation of intra- and inter-fraction movement of the cervix during intensity modulated radiation therapy. Radiother Oncol 2011;98:347-51. [Crossref] [PubMed]
- van de Bunt L, van der Heide UA, Ketelaars M, et al. Conventional, conformal, and intensity-modulated radiation therapy treatment planning of external beam radiotherapy for cervical cancer: The impact of tumor regression. Int J Radiat Oncol Biol Phys 2006;64:189-96. [Crossref] [PubMed]
- Beadle BM, Jhingran A, Salehpour M, et al. Cervix regression and motion during the course of external beam chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys 2009;73:235-41. [Crossref] [PubMed]
- Yamamoto R, Yonesaka A, Nishioka S, et al. High dose three-dimensional conformal boost (3DCB) using an orthogonal diagnostic X-ray set-up for patients with gynecological malignancy: a new application of real-time tumor-tracking system. Radiather Oncol 2004;73:219-22. [Crossref] [PubMed]
- Fuller DB, Naitoh J, Lee C, et al. Virtual HDR CyberKnife treatment for localized prostatic carcinoma: dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys 2008;70:1588-97. [Crossref] [PubMed]
- King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:877-82. [Crossref] [PubMed]
- Katz AJ, Santoro M, Diblasio F, et al. Stereotactic body radiotherapy for localized prostate cancer: disease control and quality of life at 6 years. Radiat Oncol 2013;8:118. [Crossref] [PubMed]
- Loblaw A, Cheung P, D'Alimonte L, et al. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: toxicity, biochemical, and pathological outcomes. Radiather Oncol 2013;107:153-8. [Crossref] [PubMed]
- Boike TP, Lotan Y, Cho LC, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol 2011;29:2020-6. [Crossref] [PubMed]
- Boda-Heggemann J, Mennemeyer P, Wertz H, et al. Accuracy of ultrasound-based image guidance for daily positioning of the upper abdomen: an online comparison with cone beam CT. Int J Radiat Oncol Biol Phys 2009;74:892-7. [Crossref] [PubMed]
- Ahmad R, Bondar L, Voet P, et al. A margin-of-the-day online adaptive intensity-modulated radiotherapy strategy for cervical cancer provides superior treatment accuracy compared to clinically recommended margins: a dosimetric evaluation. Acta Oncol 2013;52:1430-6. [Crossref] [PubMed]
- Hansen EK, Bucci MK, Quivey JM, et al. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys 2006;64:355-62. [Crossref] [PubMed]
- Schwartz DL, Garden AS, Thomas J, et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int J Radiat Oncol Biol Phys 2012;83:986-93. [Crossref] [PubMed]
- Tanderup K, Georg D, Potter R, et al. Adaptive management of cervical cancer radiotherapy. Semin Radiat Oncol 2010;20:121-9. [Crossref] [PubMed]
- Kerkhof EM, van der Put RW, Raaymakers BW, et al. Intrafraction motion in patients with cervical cancer: The benefit of soft tissue registration using MRI. Radiother Oncol 2009;93:115-21. [Crossref] [PubMed]
- Kagei K, Tokuuye K, Okumura T, et al. Long-term results of proton beam therapy for carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys 2003;55:1265-71. [Crossref] [PubMed]
- Park HC, Shimizu S, Yonesaka A, et al. High dose three-dimensional conformal boost using the real-time tumor tracking radiotherapy system in cervical cancer patients unable to receive intracavitary brachytherapy. Yonsei Med J 2010;51:93-9. [Crossref] [PubMed]
- Matsuura K, Tanimoto H, Fujita K, et al. Early clinical outcomes of 3D-conformal radiotherapy using accelerated hyperfractionation without intracavitary brachytherapy for cervical cancer. Gynecol Oncol 2007;104:11-4. [Crossref] [PubMed]
- Mazzola R, Ricchetti F, Fiorentino A, et al. Weekly Cisplatin and Volumetric-Modulated Arc Therapy With Simultaneous Integrated Boost for Radical Treatment of Advanced Cervical Cancer in Elderly Patients: Feasibility and Clinical Preliminary Results. Technol Cancer Res Treat 2017;16:310-5. [Crossref] [PubMed]
- Haas JA, Witten MR, Clancey O, et al. CyberKnife Boost for Patients with Cervical Cancer Unable to Undergo Brachytherapy. Front Oncol 2012;2:25. [Crossref] [PubMed]
- Kubicek GJ, Xue J, Xu Q, et al. Stereotactic body radiotherapy as an alternative to brachytherapy in gynecologic cancer. Biomed Res Int 2013;2013:898953.
- Hsieh CH, Tien HJ, Hsiao SM, et al. Stereotactic body radiation therapy via helical tomotherapy to replace brachytherapy for brachytherapy-unsuitable cervical cancer patients - a preliminary result. Onco Targets Ther 2013;6:59-66. [Crossref] [PubMed]
- Mantz C. Stereotactic Body Radiation Therapy as a Boost Alternative for Nonmetastatic Cancer of the Cervix and Endometrium: Disease Control and Quality of Life Outcomes From a Phase 2 Trial at 3 Years’ Minimum Follow-up. Int J Radiat Oncol Biol Phys 2016;96:E286. [Crossref]
- Potter R, Haie-Meder C, Van Limbergen E, et al. Recommendations from gynaecological (GYN) GEC ESTRO working group (II): concepts and terms in 3D image-based treatment planning in cervix cancer brachytherapy-3D dose volume parameters and aspects of 3D image-based anatomy, radiation physics, radiobiology. Radiother Oncol 2006;78:67-77. [Crossref] [PubMed]
- Petereit DG, Pearcey R. Literature analysis of high dose rate brachytherapy fractionation schedules in the treatment of cervical cancer: is there an optimal fractionation schedule? Int J Radiat Oncol Biol Phys 1999;43:359-66. [Crossref] [PubMed]
- Hyun Kim T, Choi J, Park SY, et al. Dosimetric parameters that predict late rectal complications after curative radiotherapy in patients with uterine cervical carcinoma. Cancer 2005;104:1304-11. [Crossref] [PubMed]
- Clark BG, Souhami L, Roman TN, et al. The prediction of late rectal complications in patients treated with high dose-rate brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 1997;38:989-93. [Crossref] [PubMed]
- Noda SE, Ohno T, Kato S, et al. Late rectal complications evaluated by computed tomography-based dose calculations in patients with cervical carcinoma undergoing high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2007;69:118-24. [Crossref] [PubMed]
- Ferrigno R, dos Santos Novaes PE, Pellizzon AC, et al. High-dose-rate brachytherapy in the treatment of uterine cervix cancer. Analysis of dose effectiveness and late complications. Int J Radiat Oncol Biol Phys 2001;50:1123-35. [Crossref] [PubMed]
- Wong FC, Tung SY, Leung TW, et al. Treatment results of high-dose-rate remote afterloading brachytherapy for cervical cancer and retrospective comparison of two regimens. Int J Radiat Oncol Biol Phys 2003;55:1254-64. [Crossref] [PubMed]
- Fu KK, Phillips TL. High-dose-rate versus low-dose-rate intracavitary brachytherapy for carcinoma of the cervix. Int J Radiat Oncol Biol Phys 1990;19:791-6. [Crossref] [PubMed]
- Orton CG, Seyedsadr M, Somnay A. Comparison of high and low dose rate remote afterloading for cervix cancer and the importance of fractionation. Int J Radiat Oncol Biol Phys 1991;21:1425-34. [Crossref] [PubMed]
- Wang X, Liu R, Ma B, et al. High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. Cochrane Database Syst Rev 2010.CD007563. [PubMed]
- Rice TW, Adelstein DJ, Koka A, et al. Accelerated induction therapy and resection for poor prognosis stage III non-small cell lung cancer. Ann Thorac Surg 1995;60:586-91; discussion 91-2. [Crossref] [PubMed]


