Management for non-variceal upper gastrointestinal bleeding in elderly patients: the experience of a tertiary university hospital
Introduction
Upper gastrointestinal bleeding (UGIB) is a common medical emergency and an important cause of morbidity and mortality. Peptic ulcer bleeding (PUB) is the main cause of non-variceal UGIB (1,2). Non-steroidal anti-inflammatory drugs (NSAIDs), low-dose aspirin (LDA) use, and Helicobacter pylori (H. pylori) infections are the main risk factors for UGIB (3,4). It is assumed that the burden of peptic ulcers has lessened due to advancements in endoscopic techniques, reduced prevalence of H. pylori, and increased utilization of acid suppressive drug therapy (2-5). In Japan, the rate of H. pylori infection has declined in young people (6,7), but there are serious cases of comorbidities and concomitant medications such as NSAIDs and antithrombotic drugs in elderly people, so that the causes of bleeding and treatment outcomes are predicted to differ with age.
Current epidemiological data show that elderly patients tend to have comorbidities, and they experience worse outcomes than non-elderly UGIB patients (8,9). Therefore, elderly patients with UGIB should have a predicted outcome (poor prognosis, refractory to treatment, long-term hospitalization, and so on) as soon as possible on their first visit via the appropriate risk scoring system (10). Several risk scoring systems, e.g., Glasgow-Blatchford score and Rockall risk score, have been devised to identify patients with acute non-variceal UGIB who are at a high risk of poor outcomes (11,12). However, many of these scoring systems are not widely used in clinical practice due to complicated calculations. On the other hand, AIMS65 evaluates only five risk factors, which accurately predicts in-hospital mortality and length of stay, and is a very simple risk scoring system for predicting outcomes in patients with acute UGIB (Table 1) (13). In fact we reported that elderly patients over the age of 70 years had many severe comorbidities and a poor prognosis (14). Since AIMS65 sets a high risk for patients age 65 and older, we re-analyzed whether the prognosis and risk factors would be changed between the elderly group (over 65 years old) and the non-elderly group (younger than 65).

Full table
Methods
During the study period between 2003 and 2011, there were 570 UGIB cases overall consisting of 504 cases of non-variceal UGIB and 66 cases of variceal bleeding at Tottori University Hospital. The consecutive 504 cases underwent urgent endoscopic examination due to symptoms such as hematemesis, melena and revealed stigmata of the non-variceal UGIB. Before urgent endoscopic examination, in principle, we had acquired written informed consent from all patients.
These patients had their background (age, sex, comorbidities, concomitant drug, etc.), clinical information at first visit (vital sign, conscious state, endoscopic findings, cause of bleeding, etc.), treatment status (required or not required treatments including endoscopic hemostasis, interventional radiology (IVR) and surgery; could or could not complete hemostasis, etc.) and their clinical outcomes analyzed. Refractory bleeding was defined as unsuccessful endoscopic hemostasis or recurrent bleeding, and poor outcome was defined when patients died in-hospital within 30 days.
Furthermore, the relationship between AIMS65 and outcome was investigated. AIMS65 scores only five risk factors, each with one point: albumin less than 3.0 g/dL, international normalized ratio greater than 1.5, altered mental status, systolic blood pressure 90 mmHg or lower, and age over 65 years (Table 1) (13).
Statistical analysis was performed by Chi-square test or m × n (3×2) test for categorical variables. A P value <0.05 was considered statistically significant. Furthermore, sensitivity, specificity and negative predictive value (NPV) of the stratification of death risk by AIMS65 were analyzed.
Results
Patient background and characteristics
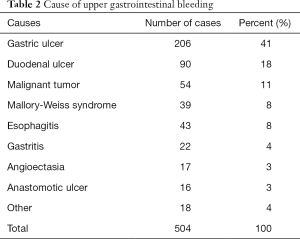
Table 2 shows the causes of UGIB in this study cohort. Gastric ulcers were the most frequent with 206 cases (41%), duodenal ulcers were seen in 90 cases (18%), and peptic ulcers occupied more than 60% when anastomotic ulcers were also added (n=312). Among the 312 cases (62%) of ulcer lesions, 197 cases fell into Forrest classification Ia, Ib, or IIa, which were considered as an absolute indication for endoscopic treatment and treated immediately. There were 29 cases in Ia, 68 in Ib and 100 in IIa. Thirty-six cases were classified as IIb, and the remainder (n=79) had “clean-based ulcers”. There were 186 NSAIDs users and 121 antithrombotic drug users. LDA was prescribed in 73 cases, warfarin potassium in 56, thienopyridine derivative agent in 25, cilostazol in 8 and heparin in 4. None took direct oral anticoagulants (DOACs).
Full table
The patient backgrounds of 504 cases were examined by separating elderly people and non-elderly people at the cut off age of 65 (Table 3). In the elderly group, complications of each comorbid diseases were significantly more frequent (P<0.00001), in particular cardiac disease, cerebrovascular disease, and orthopedic disease were substantially more frequent. In addition, antithrombotic agents and NSAIDs were also prescribed significantly more frequently in the elderly group (P<0.00001 and P<0.05, respectively). As for the frequency of the use of antithrombotic drugs, they were significantly different in LDA, warfarin, and thienopyridine derivative agent between the elderly and non-elderly group (P<0.01). Also, the elderly group had significantly more cases of onset in-hospital symptoms (P<0.001).
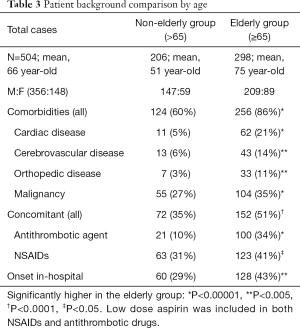
Full table
Outcome
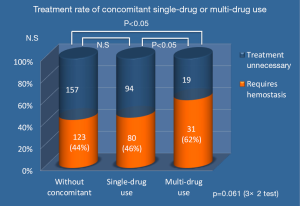
Two hundred and thirty-four cases needed hemostasis; 11 cases underwent unsuccessful endoscopic treatment and 31 cases underwent re-bleeding after endoscopic hemostasis. Forty-three cases died within 30 days after initial urgent endoscopy, but only seven cases died from UGIB (Table 4). Among the study cohort, the percent of patients using multiple concomitant medications was 10%. In this multi-drug using group, the requirement of hemostatic treatment rate was significantly higher compared with the group without concomitant medication and the single-drug using group. The treatment requirement rate (excluding spraying thrombin alone) was higher (P<0.05) in a comparison of the three groups: without concomitant medication, the single agent, and multiple concomitant medications groups. The rate of hemostasis treatment tended to increase as the number of concomitant medications increased (Figure 1).
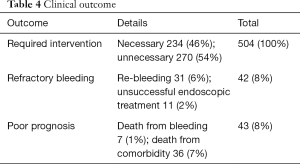
Full table
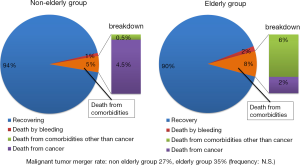
In the elderly group, the in-hospital mortality rate tended to be higher within 30 days compared to the non-elderly group (P<0.14). As a result of the analyzing prognosis by age and the cause of death, the highest cause of death was advanced malignant tumors in the non-elderly group, whereas in the elderly group the mortality rate due to severe comorbidities other than malignancy was higher (Figure 2).
Relationship between AIMS65 and the outcome
AIMS65 evaluated the five risk factors as described above, with equal weight given for each risk factor, ranged 0–5 (Table 1). In the original article, a score of 0–1 is categorized as low-risk, and a score of 2 or more is categorized as high-risk. Employing AIMS65, UGIB cases over 65 years old have at least a score of 1 (age >65).
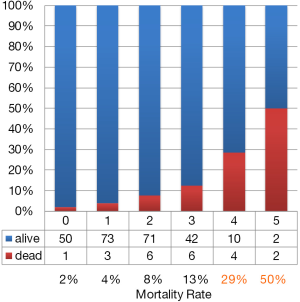
Among the patients examined, only 270 cases were capable of being studied using AIMS65. Albumin and PT-INR are necessary to evaluate AIMS65, but either or both of these two items were not measured in the remaining cases. In the 270 cases in which AIMS65 was calculated, 143 (53%) cases were stratified into high-risk, 53 cases had clinical intervention performed, and 10 cases (8%) had refractory bleeding. In the 127 low-risk patients, the NPV of refractory bleeding was 92% (117/127). On the other hand, the mortality rate in the low-risk patients was 3% (4/127) and the NPV of a poor outcome was 97% (123/127) (Table 5). A higher AIMS65 score was associated with a higher mortality rate. Especially in the 18 cases with a score of 4–5; 6 (33%) cases died from comorbidities (Figure 3).

Full table
Discussion
This study examined urgent endoscopy in our department, not only in patients who came in as emergency outpatients due to hematemesis and/or melena, but also patients with UGIB, which occurred in-hospital, were included. There was also the specialty of a university hospital. In total, 43% in the elderly group and 29% in the non-elderly group had conditions that were onset in-hospital, and the incidence of hospitalization was significantly higher in the elderly group. There are several reports about UGIB that show that those who develop it during hospitalization have a poorer prognosis than outpatients (1,15). The prevalence of all comorbidity diseases, such as cardiac disease, cerebrovascular disease, orthopedic disease and the combination rate of antithrombotic drugs/NSAIDs were also significantly higher in the elderly group, as expected. On the other hand, in both groups, the tumor burden ratio had no significant difference, but the study population could have a facility bias.
The necessity of hemostasis treatment was judged by the Forrest classification of endoscopic findings (16), and the endoscopic treatment was indicated in the cases of principle Ia, Ib, IIa. In this study, 312 cases (62%) had ulcer lesions, of which 197 cases had a Forrest classification of Ia, Ib, or IIa, which was seen as an absolute indication for treatment, 36 cases were classified as IIb, and the remainder had “clean-based ulcers”. Many of the diseases other than ulcers (such as UGIB originating from gastritis, esophagitis, or malignant tumors, etc.) did not require hemostasis. As a result, 46% of the cases required hemostasis treatment. The results were nearly equivalent to prior reported frequencies (1,2).
There were 42 cases (8%) of refractory bleeding (unsuccessful endoscopic hemostasis or recurrent bleeding) in required hemostasis cases. Of these, seven bleeding related deaths existed. The cause of bleeding related to death was in the case that the patient could not tolerate hemostasis treatment due to poor general condition, and/or massive bleeding from cancer and the large blood vessel (artery). Although it has been reported, in cases of major bleeding in which the source of bleeding cannot be confirmed, computed tomography (CT) angiography and/or IVR should be performed as soon as possible for the determination of the hemostasis by IVR and surgery promptly performed (17-19). Although not yet recommended by the international guideline (10) and the latest guidelines in Japan (20,21), the usefulness of non-contrast and contrast CT for UGIB has also been shown (22).
There is no doubt that NSAIDs and LDA raise the frequency of ulcers (3-5), as shown in this study, but there was no significant difference in the frequency of necessity of hemostasis treatment when simply examined with or without concomitant medication. However, in the group of patients using two or more concomitant medications (the multi-drug group), the treatment requiring rate was significantly higher than not only that of the group without concomitant medication but also that of the group using a single agent (the single-drug group). In Japan, proton-pump inhibitor (PPI) is recommended in the latest guidelines for the prevention of NSAIDs ulcers, especially for relapse under LDA administration. Consensus reports that PPI should be administered depending on the case (the elderly, anticoagulant therapy, administration example of two or more antithrombotic drugs, etc.) have been proposed according to cases even with oral antithrombotic drugs alone (23,24). On the other hand, there have been reports that histamine 2 receptor antagonists (H2RA) and certain mucosal protective agents have a preventive effect against NSAIDs ulcers (25,26). On the contrary, PPI has also been reported as a possible exacerbating factor for NSAIDs-induced small intestinal ulcers (27,28). It is known (3) that the risk of bleeding further increases as the number of concomitant medications increases, as suggested in this study. In total, PPI administration is considered essential for multiple concomitant drugs users.
It is extremely important to evaluate UGIB patients in acute stages with a risk scoring system on their first visit. The guidelines for UIGB recommend early risk stratification into low and high-risk categories for re-bleeding and mortality by using a risk scoring system (10). The most consistently reported predictors of mortality and re-bleeding in non-variceal UGIB have been age, number of co-morbid conditions and hemodynamic instability. Several risk scoring systems have been devised, particularly the Glasgow-Blatchford score and the Rockall risk score, the two well known risk scoring systems, have been used for predicting outcomes in patients with UGIB (11,12). Both the systems were sensitive with regard to the prediction of the necessity of hemostasis but seemed complicated in the emergent settings. The number of patients stratified into low-risk based on these scores was small in the current study. By comparison, the AIMS65 score, which accurately could predict in-hospital mortality and length of stay, is a very simple risk score for predicting outcomes in acute UGIB cases (13) (Table 1) in particular problems in elderly people. AIMS65 has no item that directly scores advanced malignant disease, serious heart disease, renal dysfunction requiring dialysis, etc. On the other hand, even with a low-score case, there might be the possibility of death due to underlying diseases. Although this is the limit of AIMS65, many patients who died even with low scores could predict their prognosis from the existence of comorbidities that obviously have a poor prognosis. On the contrary, despite not reflecting on the above medical history, it is worthwhile to predict the higher mortality rate in relation to a higher AIMS65 score.
Conclusions
In the elderly who were prescribed two or more among NSAIDs, antiplatelet agents and anticoagulation agents, hemostatic treatment rates were significantly higher. The most significant risk for poor outcome in the elderly was severe comorbidities. The elderly patients with severe coexisting diseases, in particular multiple antithrombotic agents or NSAIDs combined users, should prevent UGIB by using a PPI. We recommend that elderly cases of UGIB should be given a poor outcome estimation as soon as possible via the risk scoring system AIMS65.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Since this research was retrospective observational study, it is not consulted by the IRB. However, written informed consent was obtained from all patients before urgent endoscopy.
References
- Enestvedt BK, Gralnek IM, Mattek N, et al. An evaluation of endoscopic indications and findings related to nonvariceal upper-GI hemorrhage in a large multicenter consortium. Gastrointest Endosc 2008;67:422-9. [Crossref] [PubMed]
- Barkun A, Sabbah S, Enns R, et al. The Canadian Registry on Nonvariceal Upper Gastrointestinal Bleeding and Endoscopy (RUGBE): Endoscopic hemostasis and proton pump inhibition are associated with improved outcomes in a real-life setting. Am J Gastroenterol 2004;99:1238-46. [Crossref] [PubMed]
- García Rodríguez LA. Nonsteroidal antiinflammatory drugs, ulcers and risk: a collaborative meta-analysis. Semin Arthritis Rheum 1997;26:16-20. [Crossref] [PubMed]
- Sung JJ, Kuipers EJ, El-Serag HB. Systematic review: the global incidence and prevalence of peptic ulcer disease. Aliment Pharmacol Ther 2009;29:938-46. [Crossref] [PubMed]
- Loperfido S, Baldo V, Piovesana E, et al. Changing trends in acute upper-GI bleeding: a population-based study. Gastrointest Endosc 2009;70:212-24. [Crossref] [PubMed]
- Fujisawa T, Kumagai T, Akamatsu T, et al. Changes in seroepidemiological pattern of Helicobacter pylori and hepatitis A virus over the last 20 years in Japan. Am J Gastroenterol 1999;94:2094-9. [Crossref] [PubMed]
- Kawai T, Yamamoto K, Fukuzawa M, et al. Helicobacter pylori infection and reflux esophagitis in young and middle-aged Japanese subjects. J Gastroenterol Hepatol 2010;25 Suppl 1:S80-5. [Crossref] [PubMed]
- Ohmann C, Imhof M, Ruppert C, et al. Time-trends in the epidemiology of peptic ulcer bleeding. Scand J Gastroenterol 2005;40:914-20. [Crossref] [PubMed]
- Lau JY, Sung J, Hill C, et al. Systematic review of the epidemiology of complicated peptic ulcer disease: incidence, recurrence, risk factors and mortality. Digestion 2011;84:102-13. [Crossref] [PubMed]
- Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med 2010;152:101-13. [Crossref] [PubMed]
- Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet 2000;356:1318-21. [Crossref] [PubMed]
- Rockall TA, Logan RF, Devlin HB, et al. Risk assessment after acute upper gastrointestinal haemorrhage. Gut 1996;38:316-21. [Crossref] [PubMed]
- Saltzman JR, Tabak YP, Hyett BH, et al. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc 2011;74:1215-24. [Crossref] [PubMed]
- Kawaguchi K, Yashima K, Murawaki Y. The risk factor of poor outcome for elderly patient with nonvariceal upper gastrointestinal bleeding. The Japanese Society of Geriatric Gastroenterology 2014;17:22-8.
- Marmo R, Koch M, Cipolletta L, et al. Predicting mortality in patients with in-hospital nonvariceal upper GI bleeding: a prospective, multicenter database study. Gastrointest Endosc 2014;79:741-9. [Crossref] [PubMed]
- Forrest JA, Finlayson ND, Shearman DJ. Endoscopy in gastrointestinal bleeding. Lancet 1974;2:394-7. [Crossref] [PubMed]
- Jaeckle T, Stuber G, Hoffmann MH, et al. Detection and localization of acute upper and lower gastrointestinal (GI) bleeding with arterial phase multi-detector row helical CT. Eur Radiol 2008;18:1406-13. [Crossref] [PubMed]
- Choi YJ, Kim KS, Suh GJ, et al. Diagnostic accuracy and implementation of computed tomography angiography for gastrointestinal hemorrhage according to clinical severity. Clin Exp Emerg Med 2016;3:69-74. [Crossref] [PubMed]
- McPherson SJ, Sinclair MT, Smith NC. Severe Gastrointestinal Haemorrhage: Summary of a National Quality of Care Study with Focus on Radiological Services. Cardiovasc Intervent Radiol 2017;40:223-30. [Crossref] [PubMed]
- Satoh K, Yoshino J, Akamatsu T, et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2015. J Gastroenterol 2016;51:177-94. [Crossref] [PubMed]
- Fujishiro M, Iguchi M, Kakushima N, et al. Endoscopic clinical practice guidelines for non-varicose upper gastrointestinal bleeding. Gastroenterologiocal Endscopy 2015;57:1648-66.
- Miyaoka Y, Amano Y, Ueno S, et al. Role of enhanced multi-detector-row computed tomography before urgent endoscopy in acute upper gastrointestinal bleeding. J Gastroenterol Hepatol 2014;29:716-22. [Crossref] [PubMed]
- Bhatt DL, Scheiman J, Abraham NS, et al. ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. Am J Gastroenterol 2008;103:2890-907. [Crossref] [PubMed]
- Abraham NS, Hlatky MA, Antman EM, et al. ACCF/ACG/AHA 2010 expert consensus document on the concomitant use of proton pump inhibitors and thienopyridines: a focused update of the ACCF/ACG/AHA 2008 expert consensus document on reducing the gastrointestinal risks of antiplatelet therapy and NSAID use. A Report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. J Am Coll Cardiol 2010;56:2051-66. [Crossref] [PubMed]
- Taha AS, McCloskey C, Prasad R, et al. Famotidine for the prevention of peptic ulcers and oesophagitis in patients taking low-dose aspirin (FAMOUS): a phase III, randomised, double-blind, placebo-controlled trial. Lancet 2009;374:119-25. [Crossref] [PubMed]
- Park SH, Cho CS, Lee OY, et al. Comparison of Prevention of NSAID-Induced Gastrointestinal Complications by Rebamipide and Misoprostol: A Randomized, Multicenter, Controlled Trial-STORM STUDY. J Clin Biochem Nutr 2007;40:148-55. [Crossref] [PubMed]
- Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology 2011;141:1314-22. [Crossref] [PubMed]
- Fujimori S, Takahashi Y, Tatsuguchi A, et al. Omeprazole increased small intestinal mucosal injury in two of six disease-free cases evaluated by capsule endoscopy. Dig Endosc 2014;26:676-9. [Crossref] [PubMed]