Tips for safety in endoscopic submucosal dissection for colorectal tumors
Introduction
In Japan, endoscopic submucosal dissection (ESD) becomes one of standard therapies for large colorectal tumors (1-6). Recently, the efficacy of ESD has been reported all over the world (7). However, it is still difficult even for Japanese experts in some situations. Colonic characteristics such as the thinness of the colonic wall and winding shape make ESD difficult. Right-sided location, large tumor size, and high degree of fibrosis and so on have been previously identified as risk factors for incomplete resection and perforation of colorectal ESD (8-10). However, improvements on ESD devices, suitable strategies, and increase of operators’ experiences enable us to solve these problems (6,9). In this chapter, we introduce recent topics about various difficult factors of colorectal ESD and the tips for treating them.
Our overall ESD results and principles of ESD
Our overall therapeutic results of 1,001 ESD cases are given in Table 1. Mean age of the group was 68.1±10.5 years old and 564 were male (56.3%). Five-hundred five (50.5%), 203 (19.9%), and 293 (29.2%) tumors were located in the right-sided colon (cecum to transvers colon), left-sided colon (descending colon to sigmoid colon) and rectum, respectively. While the mean tumor size was 31.0±14.9 mm, 821 (83.1%) were non-polypoid tumors. The rate of en bloc resection was 91.9% (920/1,001). Procedural perforation and delayed perforation rates were 3.2% (32/1,001) and 0.1 (1/1,001). Postoperative hemorrhage rate was 1.8% (18/1,001). Histologically, there were 393 (40.0%), 418 (42.6%), and 124 (12.6%) cases of adenomas, Tis, and T1 cancers, respectively.
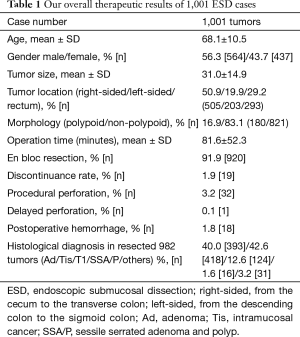
Full table
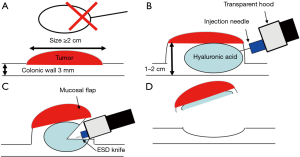
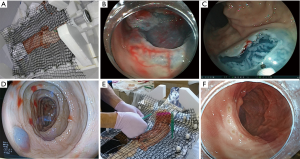
Endoscopic mucosal resection (EMR) is performed as one of standard therapies for colorectal polyps in the world. However, EMR has a limitation with respect to the size of the snare (Figure 1A). Thus, it is difficult to achieve en bloc resection by EMR for a colorectal tumor ≥20 mm in size (11,12). On the other hand, ESD enables us to achieve en bloc resection for colorectal polyps ≥20 mm in size. However, we have to pay attention about perforation in colorectal ESD because colorectal wall thickness is only about 3 mm. Special injection solution such as hyaluronic acid is used for getting long-lasting high submucosal elevation and it makes colorectal wall thicker (1–2 cm) (Figure 1B) (13,14). Transparent hood is fitted on the tip of the endoscope for ESD. After injection, mucosal incision is performed, followed by submucosal dissection. According to transparent hood and appropriate dissection, we can make a mucosal flap and go below the tumor and the flap (Figure 1C). Below the mucosal flap, the endoscope becomes stable and submucosal dissection becomes easier by submucosal traction. Finally, the tumor is resected en bloc (Figure 1D).
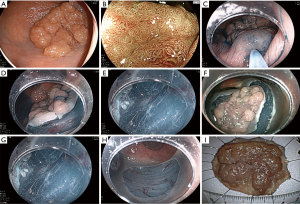
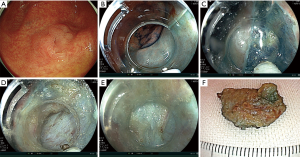
We introduce a standard ESD case. At first, we perform a pre-check colonoscopy before ESD for the accurate diagnosis (Figure 2A). Regularly, we adopt magnifying observation using narrow band imaging, blue laser imaging, and chromoendoscopy (Figure 2B) (15-19). We also determine the status of manipulation about endoscope. For cases with severely difficult manipulation, we give up to use regular endoscope and use double balloon endoscope (Fujifilm Co., Tokyo, Japan) (20). A lower gastrointestinal endoscope with a single channel (EC-L600ZP, Fujifilm Medical Co., Tokyo, Japan; PCF-H290I, Olympus Co., Tokyo, Japan) is used generally. Mucosal injection is performed to the anal side of the tumor and a partial mucosal incision is performed with ESD knife (Figure 2C,D). Then, submucosal dissection is performed for making a mucosal flap. After making a flap, we can go below the tumor and flap (Figure 2D,E). Half to 2/3 of dissection is performed followed by full circumferential mucosal incision (Figure 2F). Next, submucosal dissection is performed from the anal side again and the tumor is resected en bloc finally (Figure 2G-I).
The injection solution is prepared with 0.4% hyaluronic acid solution (Mucoup, Boston Scientific Co., Tokyo, Japan and Seikagaku Co., Tokyo, Japan) including a small amount of 0.2% indigocarmine (final concentration: 0.06% indigocarmine) (Figure 3A) (21). Injection of hyaluronic acid is administered with a 25-gauge high flow needle (TOP Co., Tokyo, Japan) (Figure 3B). Hyaluronic acid is sticky and the injection pressure is higher than saline (13,14), thus, we use high flow needle. CO2 insufflation is used for preventing severe abdominal fullness and minimizing the difficulty of manipulation (Figure 3C) (22). The VIO300D high-frequency generator (Erbe Elektromedizin, Tubingen, Germany) is also used for accurate incision, dissection, and hemostat (Figure 3D). Our routine ESD procedure is performed with a short-tipped ESD knife such as the Flush knife BT-S 2.0 mm (Fujifilm Co., Tokyo, Japan) (Figure 3E) (4). The Clutch cutter 3.5 or 5.0 mm (Fujifilm Co., Tokyo, Japan), a grasping scissor knife, was also used in difficult situations (23).

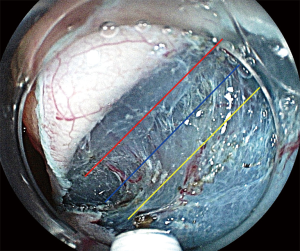
The goal of ESD is to achieve en bloc resection of large tumor for accurate histological diagnosis. Appropriate dissection depth (blue line) is important for that (Figure 4). Too shallow dissection depth makes resected specimens coagulated and damaged and it makes histological evaluation difficult. Oppositely, too deep dissection depth (yellow line) occurs procedural perforation. Especially, with respect to histological evaluation of T1 cancer, enough submucosal dissection (blue line) is needed and shallow dissection depth (yellow line) may happen vertical margin positive and incomplete resection for it (Figure 5).
Difficult factors of colorectal ESD
Difficulties of ESD are related with longer procedure, incomplete en bloc resection, and perforation (6-10). For preventing these problems, it is important to predict difficult cases. Right-sided colon tumors, fibrosis, poor endoscopic operability and deep submucosal invasion are reported to be significantly associated with incomplete en bloc resection (6,24). We previously analyzed difficult factors of colorectal ESD using 405 consecutive colorectal ESD cases performed only by an expert in our institution (25). In multivariate analyses, severe fibrosis (OR: 26.395, 95% CI: 6.587–105.764, P<0.001), difficult manipulation (OR: 4.575, 95% CI: 1.200–17.436, P=0.026), and tumor size ≥50 mm (OR: 4.452, 95% CI: 1.061–18.688), were significantly related with incomplete en bloc resection (Table 2). So, we have to pay attention to cases with these factors. In tumor sizes ≥50 mm, we consider two difficult points. First, large tumors need a more complicated strategy. In our institution, for tumor ≥50 mm, oral-side approach theory is adopted because anal-side approach sometimes makes oral side approach more difficult. After oral-side mucosal incision, anal-side incision and dissection are followed. Second, ESD for a large tumor has more complications such as perforation and perioperative hemorrhage than that for tumors that are <50 mm in size (5). Thus, a suitable strategy and considerable expertise should be considered when performing ESD for large tumors.
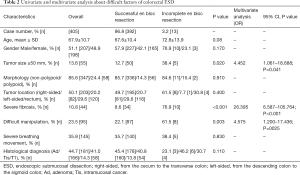
Full table
On the other hand, factors such as large tumor size, laterally spreading tumors, right-sided colon, submucosal injection without hyaluronic acid, severe fibrosis, and deeply invasive T1 cancer were reported to be associated with a higher rate of perforation in other studies (6,8,10). Poor endoscopic operability was also reported to be related with perforation (24). The rate of poor endoscopic operability was reported as 45.3%. Poor endoscopic operability is due to tumor location, breathing movement, endoscopist’s experiences and so on. Thus, we analyzed it in detail using 405 colorectal ESD cases only performed by experts. It showed the rates of difficult manipulation and severe breathing movement were 23.5% and 35.8% (25). For cases with severe breathing movement and difficult manipulation cases, we use a scissor type knife (Clutch cutter) as it enables safe dissection in this situation. Difficult manipulation resulted in discontinuance of ESD significantly (25). Thus, cases with difficult manipulation should be predicted before ESD. With respect to risk factors of poor manipulation, tumor locations at the right-sided colon and on a flexure, longer insertion time, and severe breathing movement were reported to be associated with difficult manipulation (24,25).
Cases at the dental line are recently reported to be difficult for ESD (26) because there are lots of vessels at the location. However, we manage thick vessels during ESD with the scissor-type knife (Clutch cutter). It can grasp vessels and dissect them and can stop hemorrhage without exchange to hemostatic forceps. Cases invading ileocecal valve is also difficult for ESD (27) because, there are lots of fatty tissues around ileocecal valve. It is difficult to determine appropriate dissection line in fatty tissue. It is yellowish and not transparent so that we can’t predict the length of muscle layer from submucosal layer. Additionally, it is hardly dissected by coagulation as transparent submucosa is easily dissected. Moreover, residual liquid is supplied from ileum continuously and it makes situations worse. We think most important tips for it is strategy. Mucosal incision has to be performed at the oral ileal side first as it is applied to tumors ≥50 mm in size. Because ileum is narrow and scope control on it is poor. And ileal side is hardly approached after anal-side procedure such as injection and dissection. One more our tip for cases invading ileum is to use the scissor type knife. As we previously mentioned, it is also useful for difficult manipulation.
Tips for difficult cases
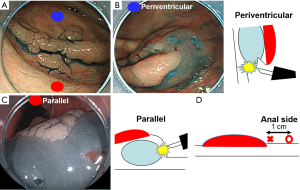
Firstly, we explain our tips about first mucosal injection using a case (Figure 6A). In this tumor, first mucosal injection can be performed at blue point or red point. If blue point is chosen, a tumor direction becomes periventricular to an endoscope (Figure 6B). It is not ideal for dissection because endoscope’s direction goes to muscle layer. On the other hand, if red point is chosen, a tumor direction becomes parallel to an endoscope (Figure 6C). It is safe and useful for dissection, because there is not muscle layer to endoscope’s direction, but high elevated submucosal layer. This theory is also useful for determining how far from the tumor the injection is performed. Too close red point (× symbol) makes tumor direction a little periventricular because part of injection goes below the tumor (Figure 6D). 1 cm anal side (〇 symbol) from the tumor makes tumor direction parallel to an endoscope.
Secondly, some traction methods should be adopted for improving difficulties of colorectal ESD (28) because regular colonoscopy has only one channel comparing to laparoscopic surgery which has more than two ports. Tapered transparent hood is regularly used for achieving traction though it is not enough effective in some cases. Clip flap method is one of easy and useful traction methods for making mucosal flap (7).
Thirdly, effective lens cleaner is necessary for colorectal ESD. Water drop adhesions (WDA) and lens cloudiness impairs the clarity of endoscopic view during colorectal ESD and are particularly annoying for endoscopists. Difficult visualization requires higher mental concentration to maintain a safe procedure, inevitably increasing strain and slowing procedure speed. Recently, we developed a novel lens cleaner and the efficacy for preventing lens cloudiness during colorectal ESD had been reported (29). The novel lens cleaner is Cleash (Fujifilm Co., Tokyo, Japan, and Nagase Medicals Co., Ltd., Hyogo, Japan) created mainly of two harmless non-ionic surfactants. It can be applied on the tip of the endoscope using swab or napkin. It showed the cloudiness of the lens decreased in 14.1% of all colorectal ESD cases with this novel lens cleaner compared to a previous lens cleaner (33.0%, P=0.002). Additionally, it could clean cloudy lens inside the colon as it was injected from the endoscopic channel into an enclosed space for 30 seconds. The space was created by pressing the endoscopic hood against the mucosa. In colorectal ESD at proximal side, we don’t want to remove an endoscope even if it becomes cloudy because re-insertion of endoscope is not easy in some cases during ESD. However, this novel lens cleaner doesn’t need endoscopists to remove an endoscope during ESD because it can clean the lens inside the colon. It is now available in Japan and we hope it will be produced to other countries in the near future.
Pocket-creation method (PCM)
The PCM is a new method for cases with severe fibrosis and one of the traction methods (Figure 7) (30). The specific feature of PCM is the creation of a large submucosal pocket using a long and tapered hood (short ST hood or ST hood: Fujifilm Co., Tokyo, Japan). PCM provides good traction with the tip of the transparent hood stretching the submucosal tissue, which facilitates submucosal dissection. Additionally, endoscope becomes stable in the pocket. So, our indications of PCM is giant colorectal tumors, laterally spreading tumor with depression, recurrent cases, and cases close to appendix, which may have severe fibrosis (9). And proximal sided tumors, which may happen difficult manipulation and severe breathing movement is also the indications of PCM.
Animal training model
In training of animal models for colorectal ESD, in vivo animal models and ex vivo animal models using harvested organs is used in the world (31). Porcine is mainly used as in vivo animal models. In vivo models having blood flow are ideal for training. However, in vivo models are expensive and inconvenient. In contrast, ex vivo animal models are inexpensive and convenient (Figure 8A). However, the weakest point of ex vivo animal models is the lack of blood flow. Control of perioperative hemorrhage is one of the most important techniques in clinical ESD. Special ex vivo animal models with blood flow has been developed in Japan and this enables ideal training for practical ESD (Figure 8B,C) (32). Based on various features, we recommend the porcine and bovine rectum for training of beginners in colorectal ESD. Regularly, imaginary lesion is made by coagulation of tip of ESD knifes, but it makes tissue damaged and hard. Thus, red ink is used for making imaginary lesion especially for EMR training (Figure 8D). In the model, ideally high submucosal elevation is achieved by injection of hyaluronic acid. The other weakness of ex vivo and in vivo animal models is the poverty of colonic fold. However, using plastic band, artificial fold is made in ex vivo model (Figure 8E,F). The shape of fold is almost similar to human one and more realistic training of ESD can be performed. These special ex vivo animal models are produced by Boston Scientific Japan (Tokyo, Japan), and so far they are only available in Japan. We hope it will be produced in other countries. This kind of animal model makes ESD more standardized all over the world.
Summary
We introduce recent topics about our tips about colorectal ESD in this chapter. There are some difficult aspects for it. Appropriate strategy, devices, and prediction of difficult cases are important for accomplishing en bloc resection without complications.
Acknowledgements
The authors thank all members of the Department of Molecular Gastroenterology and Hepatology, Kyoto Prefectural University of Medicine, for helping with this study.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yamamoto H, Kawata H, Sunada K, et al. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy 2003;35:690-4. [Crossref] [PubMed]
- Yoshida N, Wakabayashi N, Kanemasa K, et al. Endoscopic submucosal dissection for colorectal tumors: technical difficulties and rate of perforation. Endoscopy 2009;41:758-61. [Crossref] [PubMed]
- Tanaka S, Oka S, Kaneko I, et al. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc 2007;66:100-7. [Crossref] [PubMed]
- Toyonaga T, Man-i M, Chinzei R, et al. Endoscopic treatment for early stage colorectal tumors: the comparison between EMR with small incision, simplified ESD, and ESD using the standard flush knife and the ball tipped flush knife. Acta Chirurgica Iugoslavica 2010;57:41-6. [Crossref] [PubMed]
- Saito Y, Uraoka T, Yamaguchi Y, et al. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc 2010;72:1217-25. [Crossref] [PubMed]
- Isomoto H, Nishiyama H, Yamaguchi N, et al. Clinicopathological factors associated with clinical outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms. Endoscopy 2009;41:679-83. [Crossref] [PubMed]
- Yamamoto K, Michida T, Nishida T, et al. Colorectal endoscopic submucosal dissection: Recent technical advances for safe and successful procedures. World J Gastrointest Endosc 2015;7:1114-28. [PubMed]
- Kim ES, Cho KB, Park KS, et al. Factors predictive of perforation during endoscopic submucosal dissection for the treatment of colorectal tumors. Endoscopy 2011;43:573-8. [Crossref] [PubMed]
- Inada Y, Yoshida N, Kugai M, et al. Prediction and treatment of difficult cases in colorectal endoscopic submucosal dissection. Gastroenterol Res Pract 2013;2013:523084.
- Lee EJ, Lee JB, Choi YS, et al. Clinical risk factors for perforation during endoscopic submucosal dissection (ESD) for large-sized, nonpedunculated colorectal tumors. Surg Endosc 2012;26:1587-94. [Crossref] [PubMed]
- Walsh RM, Ackroyd FW, Shellito PC. Endoscopic resection of large sessile colorectal polyps. Gastrointest Endosc 1992;38:303-9. [Crossref] [PubMed]
- Tanaka S, Haruma K, Oka S, et al. Clinicopathological features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc 2001;54:62-6. [Crossref] [PubMed]
- Fujishiro M, Yahagi N, Kashimura K, et al. Comparison of various submucosal injection solutions for maintaining mucosal elevation during endoscopic mucosal resection. Endoscopy 2004;36:579-83. [Crossref] [PubMed]
- Yoshida N, Naito Y, Kugai M, et al. Efficacy of hyaluronic acid in endoscopic mucosal resection for colorectal tumors. J Gastroenterol Hepatol 2011;26:286-91. [Crossref] [PubMed]
- Yoshida N, Yagi N, Inada Y, et al. Ability of a novel blue laser imaging system for the diagnosis of colorectal polyps. Dig Endosc 2014;26:250-8. [Crossref] [PubMed]
- Yoshida N, Hisabe T, Inada Y, et al. The ability of a novel blue laser imaging system for the diagnosis of invasion depth of colorectal neoplasms. J Gastroenterol 2014;49:73-80. [Crossref] [PubMed]
- Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc 2016;28:526-33. [Crossref] [PubMed]
- Hayashi N, Tanaka S, Kanao H, et al. Relationship between narrow-band imaging magnifying observation and pit pattern diagnosis in colorectal tumors. Digestion 2013;87:53-8. [Crossref] [PubMed]
- Kudo S, Hirota S, Nakajima T, et al. Colorectal tumours and pit pattern. J Clin Pathol 1994;47:880-5. [Crossref] [PubMed]
- Hotta K, Katsuki S, Ohata K, et al. A multicenter, prospective trial of total colonoscopy using a short double-balloon endoscope in patients with previous incomplete colonoscopy. Gastrointest Endosc 2012;75:813-8. [Crossref] [PubMed]
- Yoshida N, Yagi N, Inada Y, et al. Prevention and management of complications of and training for colorectal endoscopic submucosal dissection. Gastroenterol Res Pract 2013;2013:287173.
- Maeda Y, Hirasawa D, Fujita N, et al. A prospective, randomized, double-blind, controlled trial on the efficacy of carbon dioxide insufflation in gastric endoscopic submucosal dissection. Endoscopy 2013;45:335-41. [Crossref] [PubMed]
- Akahoshi K, Motomura Y, Kubokawa M, et al. Endoscopic submucosal dissection of a rectal carcinoid tumor using grasping type scissors forceps. World J Gastroenterol 2009;15:2162-5. [Crossref] [PubMed]
- Hayashi N, Tanaka S, Nishiyama S, et al. Predictors of incomplete resection and perforation associated with endoscopic submucosal dissection for colorectal tumors. Gastrointest Endosc 2014;79:427-35. [Crossref] [PubMed]
- Yoshida N, Fernandopulle N, Murakami T, et al. Difficult manipulation is one of the risk factors for incomplete resection in colorectal endoscopic submucosal dissection. J Gastroenterol Hepatol Res 2016;5:2070-6. [Crossref]
- Imai K, Hotta K, Yamaguchi Y, et al. Safety and efficacy of endoscopic submucosal dissection of rectal tumors extending to the dentate line. Endoscopy 2015;47:529-32. [PubMed]
- Yoshizaki T, Toyonaga T, Tanaka S, et al. Feasibility and safety of endoscopic submucosal dissection for lesions involving the ileocecal valve. Endoscopy 2016;48:639-45. [Crossref] [PubMed]
- Tsuji K, Yoshida N, Nakanishi H, et al. Recent traction methods for endoscopic submucosal dissection. World J Gastroenterol 2016;22:5917-26. [Crossref] [PubMed]
- Yoshida N, Naito Y, Hirose R, et al. Risk of lens cloudiness during colorectal endoscopic submucosal dissection & ability of a novel lens cleaner to maintain and restore endoscopic view. Dig Endosc 2015;27:609-17. [Crossref] [PubMed]
- Hayashi Y, Miura Y, Yamamoto H. Pocket-creation method for the safe, reliable, and efficient endoscopic submucosal dissection of colorectal lateral spreading tumors. Dig Endosc 2015;27:534-5. [Crossref] [PubMed]
- Yoshida N, Fernandopulle N, Inada Y, et al. Training methods and models for colonoscopic insertion, endoscopic mucosal resection, and endoscopic submucosal dissection. Dig Dis Sci 2014;59:2081-90. [Crossref] [PubMed]
- Yoshida N, Yagi N, Inada Y, et al. Possibility of ex vivo animal training model for colorectal endoscopic submucosal dissection. Int J Colorectal Dis 2013;28:49-56. [Crossref] [PubMed]


