Circulating biomarkers in epithelial ovarian cancer diagnosis: from present to future perspective
Introduction
Ovarian cancer (OC) represents the most lethal female reproductive tract malignancy worldwide (1).
Current strategies and methods for OC detection include pelvic examination and transvaginal ultrasonography (US), usually performed in symptomatic subjects (2). However, signs and symptoms are mostly nonspecific (i.e., dyspepsia, bloating, early satiety, gas pains, backache) and often evident only in advanced stages (3).
Additionally, it is often difficult to discriminate between malignant and benign ovarian masses and this differential diagnosis is frequently made only after invasive histological examination.
As a consequence, nearly 70% of patients are diagnosed with advanced disease (stage III or IV) with a 5-year survival rate lower than 30% (4). Contrarily, patient’s prognosis is usually excellent if diagnosis is made in early stages.
From the discovery of CA125 in the early 80’s (5), several studies have been performed to identify and validate new single biomarkers or panels of biomarkers with high clinical sensitivity and specificity in early stages.
Despite different approaches have been used for biomarker discovery and characterization, most successes were achieved thanks to progress in proteomics and to the development of high-throughput technologies (6).
The large group of biomarkers investigated includes different molecules as cytokines, acute phase reactants growth factors, proteases, hormones and coagulation factors. However, notwithstanding apparently encouraging successes, a bridge between basic research and clinical practice (named translational oncology) (7,8) has not yet been built.
To date, only two markers [CA125 and Human Epididymis protein 4 (HE4)] have been approved by the FDA for monitoring treatment and detecting disease recurrence (9).
Since in the last decade another field of great interest has been epigenetics (DNA methylation, histone modifications and the expression of noncoding RNAs), several researchers tried to identify epigenetic biomarkers useful for OC detection. To date, no single biomarker displays high sensitivity and specificity to detect early OC and the implementation of a panel of epigenetic biomarkers is not yet feasible in clinical practice (10).
In the present review we provide an overview of the main characteristics of traditional biomarkers (i.e., CA125 and HE4) and we summarize the current knowledge regarding emerging epigenetic biomarkers [i.e., microRNAs (miRs)].
CA125
Currently, CA125, also known as mucin 16 or MUC16, a transmembrane glycoprotein produced by coelomic epithelium, is routinely used in the clinical practice and remains the only recommended serum marker to monitor the response to therapy and to confirm relapse (11,12).
However, several limits characterize this biomarker. Firstly, some OC histotypes do not release this mucin. In particular non-epithelial tumors (i.e., germ cells and sex cord-stromal tumors) do not constitutively express this glycoprotein, or only express low levels of the marker (13,14). Moreover, between epithelial OC, the release of CA125 is high in serous tumors, but lower in mucinous cancers (15). Accordingly, since also up to 20% of epithelial OC fail to express significant levels CA125 (16), it shows low sensitivity and specificity in early stages of disease. Conflicting results have been reported about the correlation between disease stage and preoperative serum concentrations of CA125: some studies (17-19) described a significant correlation between preoperative CA125 levels and International Federation of Gynecology and Obstetrics (FIGO) stage. On the other hand, other studies reported that CA125 expression is more strongly associated with OC subtype than with stage (20). Inconsistent results exist also about the preoperative and pre-chemotherapy prognostic and predictive value of serum CA125 concentrations (21-23).
CA125 displays a low specificity, by rising in several non-ovarian malignancies including cervix, breast, colon, pancreatic, lung, gastric and liver cancers (24,25). Increased CA125 levels are also observed in benign or malignant diseases affecting pleura, pericardium and peritoneum, that derive from coelomic epithelium (26) and in several pelvic diseases including endometriosis, ovarian cysts, pelvic inflammatory disease, myomas of the uterus and salpingitis, as well as non-gynecologic diseases including cirrhosis, ascites, peritoneal inflammation, pleuritis/pericarditis, pancreatitis, renal failure, liver disease (27). Since CA125 can also be expressed at the surface of inflammatory cells, high concentrations can be found in rheumatoid arthritis, scleroderma, lupus, and Sjögren’s syndrome (28). Both pregnancy and menstrual cycle can modify CA125 levels (29,30) and cyclic combined hormone replacement therapy (HRT) might also be associated with increased levels of CA125 (31). Finally, Caucasian women show higher concentrations than African and Asian women (32).
HE4
The development of sensitive and fast protein microarray and high-throughput mass spectrometry (MS) techniques has led to the discovery of several candidate biomarkers highly expressed in OC compared to healthy controls (8,33). However, the specificity of candidate biomarkers must be successively assessed in the verification phase and finally both the sensitivity and specificity should be evaluated in a clinical setting (34).
Between the large number of proteins investigated in OC, the only really introduced in clinical practice is HE4 (9).
HE4 is a 13 KDa protein coded by the gene WFDC2 which maps on human chromosome 20q12-13.1. Its mature glycosylate secretory form is approximately 25 KDa and consists of a single peptide and two whey acidic protein (WAP) domains containing a “four disulfide core” encompassing eight cysteine residues (35).
Schummer et al. demonstrated for the first time that HE4 gene was overexpressed in patients with ovarian carcinomas as compared with healthy controls (36).
A few years later, Hellström et al. measured HE4 concentrations in serum of patients with ovarian carcinoma and demonstrated that HE4 is a potentially useful biomarker for OC (37).
Several case-controls studies have been successively performed to evaluate the diagnostic performance of this biomarker by comparing it to that of the reference marker CA125 (9,38-43).
The most of these studies demonstrate that HE4 is useful in the differential diagnosis of ovarian masses (44,45). Very interestingly, it has been recently demonstrated that overexpressed HE4 has a direct biological role in the promotion of OC cells proliferation, invasion and metastasis (46).
Consequently, this biomarker, inserted with CA125 in an algorithm called Risk for Ovarian Malignancy Algorithm (ROMA), has been cleared by the Food and Drug Administration (FDA) as a diagnostic tool in the OC diagnosis (47).
In Table 1 we have summarized the main results of six meta-analysis comparing HE4 and CA125 as diagnostic markers for OC (48-53).
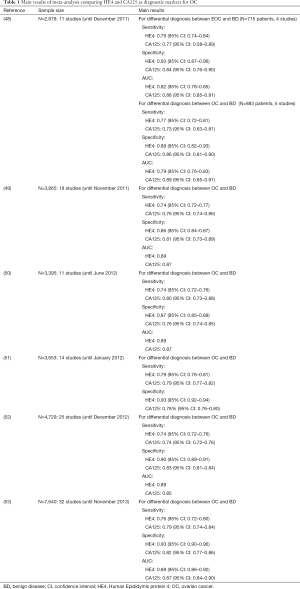
Full table
HE4 is not a perfect OC biomarker: it results increased also in other malignant neoplasms, especially of gynecologic (i.e., endometrial, tubal, vulvar cancer) and pulmonary origin (44,54-56). Accordingly, it has been proposed as a biomarker in other cancer types, in particular endometrial cancer (57-59) and lung cancer (56,60).
Moreover, many variables can affect the serum levels of this marker, as age (HE4 concentrations are reported higher in the elderly), smoke (higher by one-third in smokers than in non-smokers) and renal function (61).
Conflicting results have been reported about the relationship between HE4 values and menstrual cycle or hormonal treatment. While some authors observed higher HE4 concentrations in the ovulatory phase compared to that measured in the follicular and luteal phases (62), others affirmed that HE values are not dependent on menstrual cycle or hormonal treatment (63,64).
From single-marker diagnostics to multivariate index assays
Some multivariate index assays including the measure of one or more circulating biomarkers, have been developed as aid in the diagnostic approach to ovarian masses to determine the likelihood of malignancy (Table 2).

Full table
More than 25 years ago, Jacobs et al. proposed an algorithm, named “Risk of Malignancy Index” (RMI), by combining the values of CA125 with ultrasound and menopausal status (65). This algorithm has been used in three different versions (named RMI I, II, and III) for longtime in clinical practice in many countries (66,67).
Sensitivities and specificities for the prediction of OC among patients undergoing surgery for an adnexal mass resulted 78%, 79%, and 74%, and 87%, 81%, and 91%, for RMI I, II, and III respectively (65,68,69).
Skates et al. developed a few years later the longitudinal Bayesian “Risk of Ovarian Cancer algorithm” (ROCA) (70). This algorithm compares the CA125 profile of cases to that of HC and incorporates the known incidence of OC at a given age in calculating the risk. Accordingly, in ROCA mathematical model are included CA125 changes over time and the woman’s age.
ROCA performance has been successively investigated in several multicenter trials (71-75). In all these investigations, the reported specificity was 99.8% and the positive predictive value was between 35.1% and 37.5%.
Ova1 is a 5-protein blood biomarker panel cleared in 2009 by the FDA for the triage of patients who have a pelvic mass and need to undergo surgery (76,77). Ova1 predicts low or high risk for OC by combining the second generation CA125-II with transferrin, beta-2 microglobulin, apolipoprotein A-1, and transthyretin. In a multicenter prospective trial involving 590 women scheduled for ovarian tumor resection, Ova1 score had 96% sensitivity, 35% specificity, 40% positive-predictive value (PPV), and 95% negative-predictive value (NPV) (76,78).
After the discovery in 2009 of HE4, Moore et al. developed the Risk of Ovarian Malignancy Algorithm (ROMA) for the prediction of epithelial OC in pre-operative triaging of women affected by pelvic masses (39). This algorithm includes HE4 concentrations, CA125 values and menopausal status and is validated by setting specificity at 75% and determining sensitivity. More in details, the logistic regression model includes coefficients for the natural log (NL) of both HE4 and CA125 values and is differently calculated according to menopausal status.
Conflicting results exist about diagnostic and predictive performance of ROMA. Although some authors observed that ROMA is better than RMI in the diagnosis of EOC (79) and that diagnostic accuracy is higher using the ROMA (AUC: 0.939; 95% CI: 0.902–0.977) than using HE4 (AUC: 0.930; 95% CI: 0.891–0.969) or CA125 (AUC: 0.902; 95% CI: 0.855–0.949) (80), our and other groups failed to demonstrate ROMA superiority over HE4 alone (48,81,82).
In the study of Karlsen et al., performed on 1,218 patients with pelvic mass, the AUCs in differentiating benign from early stage OC were 0.854, 0.864, 0,897 and 0.905, for CA125, HE4, ROMA and RMI, respectively. The RMI performance (AUC: 0.945) was superior to that of CA125 (AUC: 0.925), HE4 (AUC: 0.905) and ROMA (AUC: 0.909) also in premenopausal women (83).
Lennox et al., by comparing ROMA and RMI, concluded that both these scores have poor performance in early-stage disease and low sensitivity in non-serous histologic subtypes (84).
Recently, Yanaranop et al., by enrolling in a longitudinal study 260 Thai women (n=74 affected by OC), observed that AUC in predicting OC was higher for RMI (0.876) than for ROMA, (0.862), CA125 (0.806) and HE4 (0.824). Moreover, they reported similar AUCs (0.844 and 0.856) for ROMA and RMI in premenopausal women, but RMI resulted superior to ROMA in postmenopausal women (AUCs: 0.879 and 0.840, respectively) (85).
In 2016, FDA cleared the next-generation of Ova1, named Overa, which includes CA125-II, HE4, apolipoprotein A-1, follicle stimulating hormone and transferrin (86). This new assay shows a high sensitivity but a low specificity: Ueland et al. reported a sensitivity of 94%, 89% and 91% with a specificity of 54%, 83% and 69%, for Ova1, ROMA and Overa, respectively (87).
Karlsen et al., by enrolling 809 patients with benign ovarian disease and 246 women affected by OC, developed a biomarker-based index named Copenhagen Index (CPH-I) comprising HE4, CA125 and age (88). CPH-I was then validated in eight international studies comprising 1,060 patients with benign ovarian masses and 550 patients with OC. Despite CPH-I performance resulted similar to that of ROMA and RMI (AUC: 0.960 vs. 0.954 and 0.959), this index has the advantage to be independent of ultrasound and menopausal status.
Accordingly, in a prospective study involving 1,218 women (n=252 OC), aimed to compare the performance of CA125, HE4, ROMA, CPH-I and RMI in the differentiation between benign and malignant ovarian tumor, the authors reported an AUC of 0.920 for CA125, 0.933 for HE4, 0.946 for ROMA, 0.959 for CPH-I and 0.958 for RMI (89).
However, when all ovarian malignant tumors (epithelial OC, borderline tumors, ovarian metastases and non-epithelial OC) are included in the analysis, the performances of both CPH-I and ROMA result significantly reduced (90).
Circulating miRs
miRs are active small (19–25 nt) non-coding highly conserved RNA molecules that bind specifically to, and post-transcriptionally regulate, several messenger RNAs (mRNAs) (91).
It has been demonstrated that miRs are involved in cancer biology through controlling expression of their target mRNAs to facilitate tumor growth, invasion, angiogenesis, and immune evasion (92).
The discovery that miRs circulate in the peripheral blood and can be thus measured not only invasively in tissues but also in plasma or serum, has open the possibility to use these nucleic acids as diagnostic and prognostic cancer biomarkers. Moreover, miRs have the advantage to be highly stable in plasma and serum and to be resistant to endogenous ribonuclease activity (93).
Accordingly, more than 20 studies have demonstrated that several miRs involved in different OC pathways are dysregulated (up- or down-expressed) in cancer patients (94-96).
The first study reporting the correspondence between over-expression of miRs (miR-21, miR-141, miR-200a, miR-200c, miR-200b, miR-203, miR-205, and miR-214) in OC tissue and serum-derived exosomes (97) was published in 2008.
One year later, Resnick et al. detected 21 miRs differentially expressed in serum of OC patients compared to healthy controls (96).
Different studies identified one or more miRs deregulated and thus potentially involved in OC carcinogenesis: Kan et al. demonstrated high expression levels of miR-200 family members (98), Xu et al. (99) found higher levels of miR-21, Guo et al. the up-regulation of miR-92 (100) and Zheng et al. observed the up-regulation of miR-205 and miR-483-5p and the down-regulation of let-7f in plasma of OC patients (101). In this last work let-7f and miR-205 were suggested as potential biomarkers for the early detection of EOC.
Chung and colleagues analyzed the RNA from two OC patients and a healthy control and demonstrated that 95 miRs were down-regulated and 88 miRs were up-regulated in the serum, tissue, and ascites of cancer patients (102). In the validation phase, performed by enrolling 18 patients and 12 controls, the authors observed that 5 miRNAs (miR-132, miR-26a, let-7b, miR-145, and miR-143) were markedly down-regulated in the serum from OC patients with respect to those of controls.
One year later, by using the deep sequencing technology (Solexa) and real-time PCR in serum samples of 31 OC patients, 23 patients with benign ovarian tumors and 8 control samples, Ji and colleagues demonstrated in cancer the up-regulation of miR-22, miR-93 and miR-451 and the down-regulation of miR-106b (103).
Zuberi et al. documented the high expression of miR-125b in serum of OC women and reported for miR-125b an AUC of 0.73 (95% CI: 0.64–0.81) to discriminate patients with malignant OC from healthy controls. At the best cut-off, the sensitivity and specificity were 62.3% and 77.1%, respectively (104).
By investigating 42 OC patients, 36 women diagnosed with a benign neoplasm and 23 healthy controls, Shapira et al. defined a 22-miRs profile to distinguish between OC and healthy controls and a 6-miRs profile to distinguish benign and OC patients (105).
Meng et al., by enrolling 60 EOC patients and 20 affected by benign diseases, demonstrated that the combination of miR-200a, miR-200b and miR-200c displayed a sensitivity of 83% and a specificity of 100%, to differentiate malignant from benign ovarian tumors (106).
To explore in more depth the current knowledge of miRs as potential biomarkers for OC, we suggest to read three recent reviews on this topic (107-109).
Conclusions
In the past years, a wide spectrum of serological biomarkers for OC detection has been investigated. However, a perfect and reliable biomarker (stable, highly specific and sensitive, inexpensive), is currently unavailable.
Unfortunately, despite the potential clinical utility of the proposed circulating miRs, the most of these have been validated in small cohort sizes, and at present, HE4 and CA125 remain the only biomarkers approved and applied in clinical setting.
Finally, further investigations are needed to verify diagnostic performance and to validate in large populations multi-marker panels.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- van Nagell JR Jr, Hoff JT. Transvaginal ultrasonography in ovarian cancer screening: current perspectives. Int J Womens Health 2013;6:25-33. [Crossref] [PubMed]
- Carnino F, Iskra L, Cottini M. Carcinoma of the ovary: initial symptoms and diagnostic problems. New Trends Gynaecol Obstet 1985;1:395-401.
- Holschneider CH, Berek JS. Ovarian cancer: Epidemiology, biology, and prognostic factors. Semin Surg Oncol 2000;19:3-10. [Crossref] [PubMed]
- Bast RC Jr, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983;309:883-7. [Crossref] [PubMed]
- Ye B, Gagnon A, Mok SC. Recent technical strategies to identify diagnostic biomarkers for ovarian cancer. Expert Rev Proteomics 2007;4:121-31. [Crossref] [PubMed]
- Dragani TA, Castells A, Kulasingam V, et al. Major milestones in translational oncology. BMC Med 2016;14:110. [Crossref] [PubMed]
- Santini AC, Giovane G, Auletta A, et al. Translational Research and Plasma Proteomic in Cancer. J Cell Biochem 2016;117:828-35. [Crossref] [PubMed]
- Montagnana M, Danese E, Giudici S, et al. HE4 in ovarian cancer: from discovery to clinical application. Adv Clin Chem 2011;55:1-20. [Crossref] [PubMed]
- Bijron JG, Bol GM, Verheijen RH, et al. Epigenetic biomarkers in the diagnosis of ovarian cancer. Expert Opin Med Diagn 2012;6:421-38. [Crossref] [PubMed]
- Bottoni P, Scatena R. The Role of CA 125 as Tumor Marker: Biochemical and Clinical Aspects. Adv Exp Med Biol 2015;867:229-44. [Crossref] [PubMed]
- Sölétormos G, Duffy MJ, Othman Abu Hassan S, et al. Clinical Use of Cancer Biomarkers in Epithelial Ovarian Cancer: Updated Guidelines From the European Group on Tumor Markers. Int J Gynecol Cancer 2016;26:43-51. [Crossref] [PubMed]
- Sturgeon CM, McAllister EJ. Analysis of HCG. Clinical applications and assay requirements. Ann Clin Biochem 1998;35:460. [Crossref] [PubMed]
- Begent RHJ. The value of carcinoembryonic antigen measurement in clinical practice. Ann Clin Biochem 1984;21:231. [Crossref] [PubMed]
- Høgdall EV, Christensen L, Kjaer SK, et al. CA125 expression pattern, prognosis and correlation with serum CA125 in ovarian tumor patients. From The Danish “MALOVA” Ovarian Cancer Study. Gynecol Oncol 2007;104:508-15. [Crossref] [PubMed]
- Bast RC Jr, Badgwell D, Lu Z, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer 2005;15 Suppl 3:274-81. [Crossref] [PubMed]
- But I, Gorisek B. Preoperative value of CA 125 as a reflection of tumor grade in epithelial ovarian cancer. Gynecol Oncol 1996;63:166-72. [Crossref] [PubMed]
- Rossi AC, Di Vagno G, Cormio G, et al. A retrospective study of preoperative CA 125 levels in 82 patients with ovarian cancer. Arch Gynecol Obstet 2004;269:263-5. [Crossref] [PubMed]
- Tuxen MK, Soletormos G, Dombernowsky P. Tumor markers in the management of patients with ovarian cancer. Cancer Treat Rev 1995;21:215-45. [Crossref] [PubMed]
- Köbel M, Kalloger SE, Boyd N, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 2008;5:e232. [Crossref] [PubMed]
- Díaz-Padilla I, Razak AR, Minig L, et al. Prognostic and predictive value of CA-125 in the primary treatment of epithelial ovarian cancer: potentials and pitfalls. Clin Transl Oncol 2012;14:15-20. [Crossref] [PubMed]
- Høgdall E. Cancer antigen 125 and prognosis. Curr Opin Obstet Gynecol 2008;20:4-8. [Crossref] [PubMed]
- de Bruijn HW, van der Zee AG, Aalders JG. The value of cancer antigen 125 (CA 125) during treatment and follow-up of patients with ovarian cancer. Curr Opin Obstet Gynecol 1997;9:8-13. [Crossref] [PubMed]
- Johnson CC, Kessel B, Riley TL, et al. Prostate, Lung, Colorectal and Ovarian Cancer Project Team. The epidemiology of CA-125 in women without evidence of ovarian cancer in the Prostate, Lung, Colorectal and Ovarian Cancer (PLCO) Screening Trial. Gynecol Oncol 2008;110:383-9. [Crossref] [PubMed]
- Tuxen MK. Tumor marker CA125 in ovarian cancer. J Tumor Markers Oncol 2001;16:49-68.
- Daoud E, Bodor G. CA-125 concentrations in malignant and nonmalignant disease. Clin Chem 1991;37:1968-74. [PubMed]
- Sevinc A, Adli M, Kalender ME, et al. Benign causes of increased serum CA-125 concentration. Lancet Oncol 2007;8:1054-5. [Crossref] [PubMed]
- Szekanecz E, Sándor Z, Antal-Szalmás P, et al. Increased production of the soluble tumor-associated antigens CA19-9, CA125, and CA15-3 in rheumatoid arthritis: potential adhesion molecules in synovial inflammation? Ann N Y Acad Sci 2007;1108:359-71. [Crossref] [PubMed]
- McLemore MR, Aouizerat BE, Lee KA, et al. A comparison of the cyclic variation in serum levels of CA125 across the menstrual cycle using two commercial assays. Biol Res Nurs 2012;14:250-6. [Crossref] [PubMed]
- Kobayashi F, Sagawa N, Nakamura K, et al. Mechanism and clinical significance of elevated CA 125 levels in the sera of pregnant women. Am J Obstet Gynecol 1989;160:563-6. [Crossref] [PubMed]
- Cecchi E, Lapi F, Vannacci A, et al. Increased levels of CA 125 and CA 19.9 serum tumour markers following cyclic combined hormone replacement therapy. J Clin Pharm Ther 2009;34:129-32. [Crossref] [PubMed]
- Pauler DK, Menon U, McIntosh M, et al. Factors influencing serum CA125II levels in healthy postmenopausal women. Cancer Epidemiol Biomarkers Prev 2001;10:489-93. [PubMed]
- Elzek MA, Rodland KD. Proteomics of ovarian cancer: functional insights and clinical applications. Cancer Metastasis Rev 2015;34:83-96. [Crossref] [PubMed]
- Gagnon A, Ye B. Discovery and application of protein biomarkers for ovarian cancer. Curr Opin Obstet Gynecol 2008;20:9-13. [Crossref] [PubMed]
- Clauss A, Lilja H, Lundwall A. A locus on human chromosome 20 contains several genes expressing protease inhibitor domains with homology to whey acidic protein. Biochem J 2002;368:233-42. [Crossref] [PubMed]
- Schummer M, Ng WV, Bumgarner RE, et al. Comparative hybridization of an array of 21,500 ovarian cDNAs for the discovery of genes overexpressed in ovarian carcinomas. Gene 1999;238:375-85. [Crossref] [PubMed]
- Hellström I, Raycraft J, Hayden-Ledbetter M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res 2003;63:3695-700. [PubMed]
- Havrilesky LJ, Whitehead CM, Rubatt JM, et al. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol 2008;110:374-82. [Crossref] [PubMed]
- Moore RG, McMeekin DS, Brown AK, et al. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2009;112:40-6. [Crossref] [PubMed]
- Moore RG, Brown AK, Miller MC, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol 2008;108:402-8. [Crossref] [PubMed]
- Montagnana M, Lippi G, Ruzzenente O, et al. The utility of serum human epididymis protein 4 (HE4) in patients with a pelvic mass. J Clin Lab Anal 2009;23:331-5. [Crossref] [PubMed]
- Huhtinen K, Suvitie P, Hiissa J, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer 2009;100:1315-1319. [Crossref] [PubMed]
- Montagnana M, Lippi G, Danese E, et al. Usefulness of serum HE4 in endometriotic cysts. Br J Cancer 2009;101:548. [Crossref] [PubMed]
- Karlsen NS, Karlsen MA, Høgdall CK, et al. HE4 tissue expression and serum HE4 levels in healthy individuals and patients with benign or malignant tumors: a systematic review. Cancer Epidemiol Biomarkers Prev 2014;23:2285-95. [Crossref] [PubMed]
- Granato T, Porpora MG, Longo F, et al. HE4 in the differential diagnosis of ovarian masses. Clin Chim Acta 2015;446:147-55. [Crossref] [PubMed]
- Zhu L, Zhuang H, Wang H, et al. Overexpression of HE4 (human epididymis protein 4) enhances proliferation, invasion and metastasis of ovarian cancer. Oncotarget 2016;7:729-44. [Crossref] [PubMed]
- FDA [homepage on the Internet]. U.S. Food and Drug Administration. Available online: https://www.fda.gov/ohrms/dockets/ac/08/briefing/220028-4403b1-02-proposed%20he4%20eia%20package%20insert%20%20.pdf
- Li F, Tie R, Chang K, et al. Does risk for ovarian malignancy algorithm excel human epididymis protein 4 and CA125 in predicting epithelial ovarian cancer: a meta-analysis. BMC Cancer 2012;12:258. [Crossref] [PubMed]
- Lin JY, Qin JB, Li XY, et al. Diagnostic value of human epididymis protein 4 compared with mesothelin for ovarian cancer: a systematic review and meta-analysis. Asian Pac J Cancer Prev 2012;13:5427-32. [Crossref] [PubMed]
- Lin J, Qin J, Sangvatanakul V. Human epididymis protein 4 for differential diagnosis between benign gynecologic disease and ovarian cancer: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol 2013;167:81-5. [Crossref] [PubMed]
- Ferraro S, Braga F, Lanzoni M, et al. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review. J Clin Pathol 2013;66:273-81. [Crossref] [PubMed]
- Zhen S, Bian LH, Chang LL, et al. Comparison of serum human epididymis protein 4 and carbohydrate antigen 125 as markers in ovarian cancer: A meta-analysis. Mol Clin Oncol 2014;2:559-66. [PubMed]
- Wang J, Gao J, Yao H, et al. Diagnostic accuracy of serum HE4, CA125 and ROMA in patients with ovarian cancer: a meta-analysis. Tumour Biol 2014;35:6127-38. [Crossref] [PubMed]
- Moore RG, Brown AK, Miller MC, et al. Utility of a novel serum tumor biomarker HE4 in patients with endometrioid adenocarcinoma of the uterus. Gynecol Oncol 2008;110:196-201. [Crossref] [PubMed]
- Montagnana M, Lippi G, Danese E, et al. Human Epydidimis Protein 4 (HE4): it could be useful in vulvar cancer? Clin Lab 2010;56:601-2. [PubMed]
- Zeng Q, Liu M, Zhou N, et al. Serum human epididymis protein 4 (HE4) may be a better tumor marker in early lung cancer. Clin Chim Acta 2016;455:102-6. [Crossref] [PubMed]
- Bie Y, Zhang Z. Diagnostic value of serum HE4 in endometrial cancer: a meta-analysis. World J Surg Oncol 2014;12:169. [Crossref] [PubMed]
- Hu L, Du S, Guo W, et al. Comparison of Serum Human Epididymis Protein 4 and Carbohydrate Antigen 125 as Markers in Endometrial Cancer: A Meta-Analysis. Int J Gynecol Cancer 2016;26:331-40. [Crossref] [PubMed]
- Chen Y, Ren YL, Li N, et al. Serum human epididymis protein 4 vs. carbohydrate antigen 125 and their combination for endometrial cancer diagnosis: a meta-analysis. Eur Rev Med Pharmacol Sci 2016;20:1974-85. [PubMed]
- Tang QF, Zhou ZW, Ji HB, et al. Value of serum marker HE4 in pulmonary carcinoma diagnosis. Int J Clin Exp Med 2015;8:19014-21. [PubMed]
- Ferraro S, Schiumarini D, Panteghini M. Human epididymis protein 4: factors of variation. Clin Chim Acta 2015;438:171-7. [Crossref] [PubMed]
- Anastasi E, Granato T, Marchei GG, et al. Ovarian tumor marker HE4 is differently expressed during the phases of the menstrual cycle in healthy young women. Tumour Biol 2010;31:411-5. [Crossref] [PubMed]
- Hallamaa M, Suvitie P, Huhtinen K, et al. Serum HE4 concentration is not dependent on menstrual cycle or hormonal treatment among endometriosis patients and healthy premenopausal women. Gynecol Oncol 2012;125:667-72. [Crossref] [PubMed]
- Moore RG, Plante B, Hartnett E, et al. Assessment of serum HE4 levels throughout the normal menstrual cycle. Am J Obstet Gynecol 2017;217:53.e1-53.e9.
- Jacobs I, Oram D, Fairbanks J, et al. A risk of malignancy index incorporating CA125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol 1990;97:922-9. [Crossref] [PubMed]
- Le T, Giede C, Salem S, et al. Initial evaluation and referral guidelines for management of pelvic/ovarian masses. J Obstet Gynaecol Can 2009;31:668-80. [Crossref] [PubMed]
- Clarke SE, Grimshaw R, Rittenberg P, et al. Risk of malignancy index in the evaluation of patients with adnexal masses. J Obstet Gynaecol Can 2009;31:440-5. [Crossref] [PubMed]
- Geomini P, Kruitwagen R, Bremer GL, et al. The accuracy of risk scores in predicting ovarian malignancy: a systematic review. Obstet Gynecol 2009;113:384-94. [Crossref] [PubMed]
- Tingulstad S, Hagen B, Skjeldestad FE, et al. Evaluation of a risk of malignancy index based on serum CA125, ultrasound findings and menopausal status in the pre-operative diagnosis of pelvic masses. Br J Obstet Gynaecol 1996;103:826-31. [Crossref] [PubMed]
- Skates SJ, Xu FJ, Yu YH. Toward an optimal algorithm for ovarian cancer screening with longitudinal tumor markers. Cancer 1995;76:2004-10. [Crossref] [PubMed]
- Skates SJ. Ovarian cancer screening: development of the risk of ovarian cancer algorithm (ROCA) and ROCA screening trials. Int J Gynecol Cancer 2012;22 Suppl 1:S24-6. [Crossref] [PubMed]
- Menon U, Skates SJ, Lewis S, et al. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol 2005;23:7919-26. [Crossref] [PubMed]
- Menon U, Gentry-Maharaj A, Hallett R, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol 2009;10:327-40. [Crossref] [PubMed]
- Skates SJ, Mai P, Horick NK, et al. Large prospective study of ovarian cancer screening in high-risk women: CA125 cut-point defined by menopausal status. Cancer Prev Res (Phila) 2011;4:1401-8. [Crossref] [PubMed]
- Greene MH, Piedmonte M, Alberts D, et al. A prospective study of risk-reducing salpingo-oophorectomy and longitudinal CA-125 screening among women at increased genetic risk of ovarian cancer: design and baseline characteristics: a Gynecologic Oncology Group study. Cancer Epidemiol Biomarkers Prev 2008;17:594-604. [Crossref] [PubMed]
- Ueland FR, DeSimone C, Seamon L, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol 2011;117:1289-97. [Crossref] [PubMed]
- Zhang Z, Chan D. The road from discovery to clinical diagnostics: Lessons learned from the first FDA-cleared in vitro diagnostic multivariate index assay of proteomic biomarkers. Cancer Epidemiol Biomarkers Prev 2010;19:2995-9. [Crossref] [PubMed]
- Ware Miller R, Smith A, DeSimone CP, et al. Performance of the American College of Obstetricians and Gynecologists’ ovarian tumor referral guidelines with a multivariate index assay. Obstet Gynecol 2011;117:1298-306. [Crossref] [PubMed]
- Moore RG, Jabre-Raughley M, Brown AK, et al. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol 2010;203:228.e1-6. [Crossref] [PubMed]
- Macuks R, Baidekalna I, Donina S. An ovarian cancer malignancy risk index composed of HE4, CA125, ultrasonographic score, and menopausal status: use in differentiation of ovarian cancers and benign lesions. Tumour Biol 2012;33:1811-7. [Crossref] [PubMed]
- Montagnana M, Danese E, Ruzzenente O, et al. The ROMA (risk of ovarian malignancy algorithm) for estimating the risk of epithelial ovarian cancer in women presenting with pelvic mass: is it really useful? Clin Chem Lab Med 2011;49:521-5. [Crossref] [PubMed]
- Jacob F, Meier M, Caduff R, et al. No benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical setting. Gynecol Oncol 2011;121:487-91. [Crossref] [PubMed]
- Karlsen MA, Sandhu N, Hogdall C, et al. Evaluation of HE4, CA125, Risk of Ovarian Malignancy Algorithm (ROMA) and Risk of Malignancy Index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol 2012;127:379-83. [Crossref] [PubMed]
- Lennox GK, Eiriksson LR, Reade CJ, et al. Effectiveness of the risk of malignancy index and the risk of ovarian malignancy algorithm in a cohort of women with ovarian cancer: does histotype and stage matter? Int J Gynecol Cancer 2015;25:809-14. [Crossref] [PubMed]
- Yanaranop M, Anakrat V, Siricharoenthai S, et al. Is the Risk of Ovarian Malignancy Algorithm Better Than Other Tests for Predicting Ovarian Malignancy in Women with Pelvic Masses? Gynecol Obstet Invest 2017;82:47-53. [Crossref] [PubMed]
- Coleman RL, Herzog JT, Chan DW, et al. Validation of a second-generation multivariate index assay for malignancy risk of adnexal masses. Am J Obstet Gynecol 2016;215:82.e1-82.e11. [Crossref] [PubMed]
- Ueland FR. A Perspective on Ovarian Cancer Biomarkers: Past, Present and Yet-To-Come. Diagnostics (Basel) 2017;7. [Crossref] [PubMed]
- Karlsen MA, Høgdall EV, Christensen IJ, et al. A novel diagnostic index combining HE4, CA125 and age may improve triage of women with suspected ovarian cancer - An international multicenter study in women with an ovarian mass. Gynecol Oncol 2015;138:640-6. [Crossref] [PubMed]
- Høgdall E. Approaches to the detection of ovarian cancer. Scand J Clin Lab Invest Suppl 2016;245:S49-53. [Crossref] [PubMed]
- Yoshida A, Derchain SF, Pitta DR, et al. Comparing the Copenhagen Index (CPH-I) and Risk of Ovarian Malignancy Algorithm (ROMA): Two equivalent ways to differentiate malignant from benign ovarian tumors before surgery? Gynecol Oncol 2016;140:481-5. [Crossref] [PubMed]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215-33. [Crossref] [PubMed]
- Stahlhut C, Slack FJ. MicroRNAs and the cancer phenotype: profiling, signatures and clinical implications. Genome Med 2013;5:111. [Crossref] [PubMed]
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008;105:10513-8. [Crossref] [PubMed]
- Iorio MV, Visone R, Di Leva G, et al. MicroRNA signatures in human ovarian cancer. Cancer Res 2007;67:8699-707. [Crossref] [PubMed]
- Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res 2008;14:2690-5. [Crossref] [PubMed]
- Resnick KE, Alder H, Hagan JP, et al. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 2009;112:55-9. [Crossref] [PubMed]
- Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exo- somes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol 2008;110:13-21. [Crossref] [PubMed]
- Kan CW, Hahn MA, Gard GB, et al. Elevated levels of circulating microRNA-200 family members correlate with serous epithelial ovarian cancer. BMC Cancer 2012;12:627. [Crossref] [PubMed]
- Xu YZ, Xi QH, Ge WL, et al. Identification of serum microRNA-21 as a biomarker for early detection and prognosis in human epithelial ovarian cancer. Asian Pacific Journal of Cancer Prevention 2013;14:1057-60. [Crossref] [PubMed]
- Guo F, Tian J, Lin Y, et al. Serum microRNA-92 expression in patients with ovarian epithelial carcinoma. J Int Med Res 2013;41:1456-61. [Crossref] [PubMed]
- Zheng H, Zhang L, Zhao Y, et al. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS One 2013;8:e77853. [Crossref] [PubMed]
- Chung YW, Bae HS, Song JY, et al. Detection of microRNA as novel biomarkers of epithelial ovarian cancer from the serum of ovarian cancer patient. Int J Gynecol Cancer 2013;23:673-9. [Crossref] [PubMed]
- Ji T, Zheng ZG, Wang FM, et al. Differential microRNA expression by solexa sequencing in the sera of ovarian cancer patients. Asian Pacific Journal of Cancer Prevention 2014;15:1739-43. [Crossref] [PubMed]
- Zuberi M, Khan I, Mir R, et al. Utility of Serum miR-125b as a Diagnostic and Prognostic Indicator and Its Alliance with a Panel of Tumor Suppressor Genes in Epithelial Ovarian Cancer. PLoS One 2016;11:e0153902. [Crossref] [PubMed]
- Shapira I, Oswald M, Lovecchio J, et al. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer 2014;110:976-83. [Crossref] [PubMed]
- Meng X, Müller V, Milde-Langosch K, et al. Circulating Cell-Free miR-373, miR-200a, miR-200b and miR-200c in Patients with Epithelial Ovarian Cancer. Adv Exp Med Biol 2016;924:3-8. [Crossref] [PubMed]
- Nakamura K, Sawada K, Yoshimura A, et al. Clinical relevance of circulating cell-free microRNAs in ovarian cancer. Mol Cancer 2016;15:48. [Crossref] [PubMed]
- Prahm KP, Novotny GW, Høgdall C, et al. Current status on microRNAs as biomarkers for ovarian cancer. APMIS 2016;124:337-55. [Crossref] [PubMed]
- Samuel P, Carter DR. The Diagnostic and Prognostic Potential of microRNAs in Epithelial Ovarian Carcinoma. Mol Diagn Ther 2017;21:59-73. [Crossref] [PubMed]


