Diagnosis and treatment of pulmonary cavity after liver transplantation
Introduction
Liver transplantation (LT) has become the only effective treatment for end-stage liver diseases (1). However, pulmonary infection affects chances of survival after LT (2). With timely diagnosis and treatment, most cases of pulmonary infection can be cured without affecting a patient’s chances for survival. However, some specific pulmonary infections can lead to the formation of a pulmonary cavity (PC) (3). PCs are hard to diagnose and, without accurate and timely treatment, patients carry a poor prognosis and a high risk of mortality. The main pathogenic bacteria that lead to PC formation are the Aspergillus species and Mycobacterium tuberculosis. In some cases, some other pathogenic organisms, such as pneumocystis carinii, can also cause bacterial pulmonary abscesses and pneumonia, which are necessary for the differential diagnosis of PC. Invasive pulmonary aspergillosis (IPA) is one of the most serious complications after LT, with a prevalence of 1–9.2% and mortality rates between 83–88%, and it is extremely difficult to treat (4). Pulmonary tuberculosis (TB) is another complication after LT that is difficult to diagnose at an early stage, and that has a low prevalence of being cured and a high prevalence of recurrence. These two opportunistic infections represent similar symptoms and imaging manifestations. The antibiotic therapies for these two infections could potentially cause hepatotoxicity and acute rejection. Therefore, early differential diagnosis with prompt use of specific antibiotics, along with active prevention and treatment of liver injury and acute rejection induced by antibiotics, are critical components for improving patients’ chances of survival during LT.
Methods
From January 2014 to December 2015, 159 cases of orthotopic LT were carried out in our center. All transplants were approved by the ethics committee of the First Affiliated Hospital of Nanjing Medical University. Five cases [3 males and 2 females; age, 38–61 (mean, 49.6) years] of postoperative pulmonary infection were shown as having PC formation upon CT scan (prevalence ≈3.14%). All five cases received a GM test and sputum culture and no Aspergillus or TB infection was found before LT. No pulmonary infection was indicated on a chest X-ray. The clinical findings of each patient are summarized in Table 1. Primary diseases included: chronic hepatitis B (severe) combined with hepatic encephalopathy (2 cases); primary biliary cirrhosis (1 case); hepatocellular carcinoma (1 case), and hepatitis cirrhosis (1 case). The modified piggyback method in orthotopic LT was used for all patients. After LT, an immunosuppressive therapy (tacrolimus, mycophenolate mofetil, glucocorticoid hormone) was employed to prevent transplant rejection. The post-surgery routine examination included a chest X-ray, blood cultures of blood or sputum, routine examination or biochemical test of blood, serum concentration of tacrolimus, etc. Bacterial and fungal cultivation of throat swabs and of samples of urine, sputum, and bile was conducted thrice within 1 week of transplantation; blood culture was conducted once after transplantation, followed by routine once-weekly cultivation in the intensive care unit (ICU). After surgery, antibacterial agents (ceftriaxone sodium) were used routinely without regular application of antifungal drugs. Informed consent of the clinical study was obtained from each patient, and Ethics approval (ID: 2015-SRFA-095) was obtained by Ethical Review Board of the First Affiliated Hospital of Nanjing Medical University.

Full table
Results
Case 1: the patient had endotracheal extubation at 1 week post-surgery and the time of ICU stay was 10 days. Then the patient showed cough and sputum, and he received voriconazole treatment (4 mg/kg, q12h) immediately after a diagnosis of Aspergillus infection by GM test and cultures of bronchial aspirates and sputum. The patient’s cough and pulmonary infection were alleviated after 2 weeks. However, the patient was found to have a large volume of ascites and outflow tract stenosis. Percutaneous venous balloon dilatation and stent implantation were then applied. Unfortunately, the ascites did not get better and renal failure occurred in this patient, leading to subsequent multiple organs dysfunction syndrome (MODS) and ultimately death.
Case 2: the patient had endotracheal extubation at 3 days post-surgery and was transferred to the general ward at 4 days post-surgery. He was diagnosed with Aspergillus infection using a GM test and cultures of bronchial aspirates and sputum, and he also received intravenous administration of voriconazole (4 mg/kg, q12h). After one month, the PC became much smaller and the patient’s cough got better as well. After another month, the voriconazole was administered orally (400 mg, q12h) for one more month. The patient recovered well and no recurrence was found at the fourteen-month follow-up.
Case 3: the patient had endotracheal extubation at 2 days post-surgery and stayed in the ICU for 3 days. She displayed cough, sputum and respiratory distress at one week post-surgery, and then received tracheal intubation and mechanical ventilation. The patient was diagnosed with Aspergillus infection using a GM test and cultures of bronchial aspirates and sputum, which was followed by a treatment of voriconazole (4 mg/kg, q12h) combined with caspofungin (50 mg, qd). Ten weeks later, the tracheal intubation was extubated. One of the PCs disappeared while the other became much smaller, as shown by a CT scan. Caspofungin was then discontinued. Voriconazole was intravenously administered for another 4 weeks and was then administered orally (400 mg, q12h) for 2 months. The patient’s recovery went well and no recurrence was found at the one-year follow-up.
Case 4: the tracheal intubation was removed at 4 days post-surgery and the patient was transferred to the general ward at 7 days post-surgery. He was believed to have Aspergillus infection and received a treatment of caspofungin (50 mg, qd) for 1 month. However, the patient had hemoptysis and his cough was not alleviated. TB was diagnosed using acid-fast bacilli staining of sputum smears, and then isoniazid (300 mg/d), rifampicin (450 mg/d) and pyrazinamide (2 mg/d) were continuously applied for 9 months. The PC disappeared and the patient’s recovery went well, with no recurrence at the one-year follow-up.
Case 5: the patient had endotracheal extubation at 3 days post-surgery and the time of ICU stay was 4 days. The patient was diagnosed with TB infection using acid-fast bacilli staining of sputum smears, and then received a treatment of isoniazid (300 mg/d), rifampicin (450 mg/d) and pyrazinamide (2 mg/d). The CT scan revealed that the PC decreased. The anti-TB treatment was continued for another 7 months and the PC disappeared. No recurrence was found at the one-year follow-up.
All five patients with PCs demonstrated symptoms of fever, cough, and expectoration; dyspnea was noted in one case. Time of onset of symptoms was between 3 days to 3 months after surgery. Symptoms occurred within 2 weeks after surgery for four cases, and in 3 months after surgery for one case.
Radiography of the chest revealed patchy shadows in four cases, and pulmonary nodules in two cases. PC formation was demonstrated using CT scans of the chest for all five cases. Three cases (case numbers 1–3) with Aspergillus infections were diagnosed based on cultures of bronchial aspirates or sputum, which also tested positive for the serum Aspergillus galactomannan (GM) assay. Two cases (case numbers 4 and 5) tested negative on the purified protein derivative (PPD) skin test, and tested positive for acid-fast bacilli staining of sputum smears, and they were diagnosed as having pulmonary tuberculosis.
CT of the chest
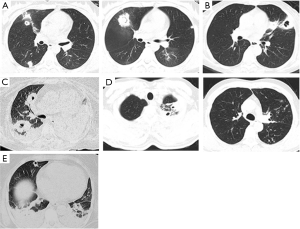
CT scans of the chest for three out of five patients demonstrated patchy, high-density shadows with or without pleural effusion. No characteristic changes were shown for the other two patients (only increased lung textures). However, PC formation was observed on CT scans of the chest for all five patients. Case 1 (Figure 1A) demonstrated multiple changes in lesions and PCs with thick walls (“air crescent sign”). Case 2 (Figure 1B) demonstrated a PC with a thick wall. Case 3 (Figure 1C) manifested multiple PCs with atelectasis. Case 4 (Figure 1D) demonstrated multiple changes in lesions with thick-walled PCs (“tree-in-bud sign”). Case 5 (Figure 1E) showed multiple thick-walled PCs with pleural effusions.
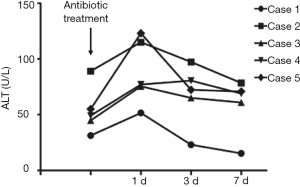
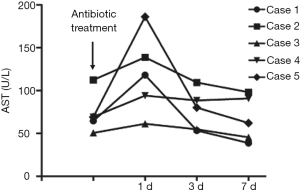
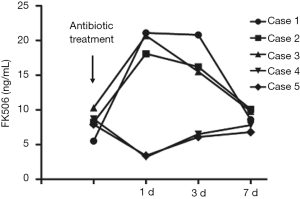
Changes in liver function and FK506 concentration during antibiotic treatment
In the three patients diagnosed with IPA, antifungal treatment with voriconazole was adopted in two cases, and voriconazole combined with caspofungin was employed for the other patient. TB patients were treated with isoniazid, rifampicin and pyrazinamide. Concentrations of alanine transaminase (ALT), aspartate transaminase (AST) and FK506 were measured on the second day of antibiotic treatment. Levels of ALT and AST in all five patients increased after the initiation of antibiotic treatment, and decreased after the application of hepatoprotective treatment, and medications were not terminated due to the toxic effects of the drugs (Figures 2,3). FK506 concentrations were increased (by ≤3.8 times) in patients 1–3 after administration of anti-Aspergillus drugs (Figure 4). FK506 concentrations of patients 4 and 5 were reduced after administration of anti-TB drugs which, generally were maintained at optimal levels after adjustment of the FK506 dose.
Prognosis
One patient (Case 1) with Aspergillus infection was treated with voriconazole. Percutaneous venous balloon dilatation and stent implantation were done because of the large volume of ascites with concurrent outflow tract stenosis after surgery. However, the volume of ascites did not decrease significantly. The pulmonary infection was relieved, but the condition was aggravated, which led to renal failure and death. The remaining four patients were cured.
Discussion
If CT scans of the chest after LT suggest a PC formation, the most common causes of PC formation are invasion of the Aspergillus species, Mycobacterium tuberculosis, and bacterial lung abscesses. Infections by the Aspergillus species and Mycobacterium tuberculosis are opportunistic infections after organ transplantation and have similarities in terms of their symptoms, imaging manifestations, and hepatotoxicity of the antibiotics used. Early differential diagnoses of these two infections followed by administration of specific antibiotics, active prevention and treatment of liver injury and acute rejection induced by antibiotics, are extremely important for improving the survival chances of liver-transplant patients.
After LT, postoperative pulmonary infection by the Aspergillus species and Mycobacterium tuberculosis is manifested as fever, cough, expectoration, hemoptysis, and PC formation, all of which have similar morphologies on CT scans. Therefore, identification of the pathogen causing PC formation after LT is difficult. Within our study cohort, one patient was suspected to have an Aspergillus infection and was subjected to 1 month of antifungal treatment, but treatment was ineffective. Subsequently, this patient was diagnosed with a Mycobacterium tuberculosis infection based on bronchoscopy, then was treated with anti-TB agents and cured.
Pulmonary infections due to Aspergillus infection and Mycobacterium tuberculosis are extremely similar on radiographies of the chest, which does not facilitate the differential diagnosis. However, these two types of infection demonstrate certain specific manifestations on CT scans. For PCs formed due to Aspergillus infection, wall thickness is uneven, and there is no flat liquid level but there is a smooth inner wall (5). For PCs formed due to invasive Aspergillus, infection develops from characteristic halo and air-crescent signs (6). Therefore, close observation of dynamic changes on CT scans helps to confirm the diagnosis of pulmonary infection by Aspergillus. PCs caused by Mycobacterium tuberculosis usually occur in the apicoposterior segment of the superior lobe and in the dorsal segment of the lower lobe (7). PCs are usually distributed on both sides of the lung in various sizes. Alternatively, there can be a single PC with uneven wall thickness, calcification of the PC wall, high density, lobules with branches around the lesion, and demonstration of the tree-in-bud sign. Kim et al. reported that among 28 cases of pulmonary infection of Mycobacterium tuberculosis in transplant patients, two-thirds were diagnosed using CT scans of the chest, and the remaining one-third could not be differentiated from pulmonary infection by Aspergillus (8). Similar to those reported cases, two patients in the present study were diagnosed based purely on CT scans: one was shown to have an Aspergillus infection based on a PC that was accompanied by the air-crescent sign, and the other was shown to have a Mycobacterium tuberculosis infection based on a PC with a bud-like sign. However, the remaining three cases could not be diagnosed based purely on CT scans. Therefore, analyses of the morphological features of PCs demonstrated using CT scans are important for distinguishing between these two types of opportunistic infections, but the diagnosis must be confirmed in combination with clinical manifestations and a specific culture of pathogens.
Calcineurin inhibitors FK506 and cyclosporine A (CsA) are metabolized by the hepatic drug-metabolizing enzyme P450 (9,10). Antifungal and anti-TB drugs are also metabolized primarily by the cytochrome P450 family, so these drugs will affect the blood concentration of immunosuppressants, leading to severe hepatotoxicity or inducing acute rejection in the liver (11,12). The first-line anti-TB drugs isoniazid and rifampicin can induce production of enzymes in liver microsomes (13). This action enhances CYP3A activity, accelerates the metabolism of FK506 or CsA, reduces the blood concentration of the drug, and induces acute rejection. Meyers et al. reported that if TB infection in LT patients is treated by isoniazid or rifampicin, the prevalence of drug-induced hepatitis is ≤83% and that the prevalence of concentration reductions in immunosuppressants and in liver-injury-induced acute rejection is 50% (14). After administration of isoniazid and rifampicin, the FK506 concentration in patients with pulmonary infection due to Mycobacterium tuberculosis decreased by 1–1.5 times. By restoring the normal concentration through increasing the drug dose, neither acute rejection reactions nor obvious hepatotoxicity were observed during anti-TB treatment. In contrast, the anti-Aspergillus agent voriconazole can markedly inhibit CYP3A activity and slow-down the metabolism of FK506 or CSA, which greatly increases the drug concentration in the blood and results in severe hepatotoxicity. Scholars have reported that voriconazole can increase the FK506 concentration by more than tenfold, which makes changing the drug dose difficult and can lead to severe hepatotoxicity. Therefore, some researchers have proposed that the use of FK506 should be stopped once voriconazole has been given and that initializing use of other immunosuppressants should occur. In the present study, the FK506 concentration increased by 2–4 times after voriconazole administration. After adjustment of the FK506 dose, its concentration was maintained at ≈10 ng/mL, which did not have a significant effect on liver function. In conclusion, the hepatotoxicity and the anti-Aspergillus and anti-TB drugs’ effect on the immunosuppressant concentration that was observed in the present study was less severe and more controllable when compared with other reports. This difference could have been due to a lack of statistical rigor due to the small size of our study cohort, but could also have been due to population differences. The conclusion of one report carried out in Hong Kong was similar to ours. Authors found that in five out of eight cases, anti-TB treatment decreased the FK506 concentration in patients, with the concentration controlled within the “ideal” therapeutic window by increasing the dose by 2–3 times and that treatment was not interrupted in any patient due to the hepatotoxicity of the anti-TB drug. During treatment, the laboratory parameters of transplanted livers were maintained within normal ranges without acute rejection (15).
Other infectious diseases that can lead to PC formation include bacterial pulmonary abscesses (16) and pneumonia due to Pneumocystis carinii (17). Pulmonary tumors (e.g., lung cancer and pulmonary lymphomas) can also lead to PC formation. These diseases were not observed in the present study.
The immune systems of LT patients are weak, and they are prone to opportunistic infections. Due to the similarities in their clinical manifestations and laboratory examinations, a prompt and accurate diagnosis is challenging, because there are no specific manifestations using radiography of the chest and because they cannot be distinguished readily from other common bacterial infections. If LT patients demonstrate symptoms of pulmonary infection (e.g., cough, expectoration, hemoptysis, fever) with PCs shown on a CT scan of the chest, pulmonary TB and Aspergillus infection should be considered. The diagnosis should be further confirmed by using a sputum culture, GM assay, PPD, and sputum smears. During treatment of TB and Aspergillus infection, changes in liver function and blood concentrations of immunosuppressants should be monitored closely, with subsequent adjustments of anti-TB drugs and antifungal agents and the dose of immunosuppressants.
Acknowledgements
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81571557) and Six Talents Peak in the Jiangsu Province of China (2013-WSW-022).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Informed consent of the clinical study was obtained from each patient, and Ethics approval (ID: 2015-SRFA-095) was obtained by Ethical Review Board of the First Affiliated Hospital of Nanjing Medical University.
References
- Schuppan D, Afdhal NH. Liver cirrhosis. Lancet 2008;371:838-51. [Crossref] [PubMed]
- Zeyneloğlu P. Respiratory complications after solid-organ transplantation. Exp Clin Transplant 2015;13:115-25. [PubMed]
- Park YS, Seo JB, Lee YK, et al. Radiological and clinical findings of pulmonary aspergillosis following solid organ transplant. Clin Radiol 2008;63:673-80. [Crossref] [PubMed]
- Singh N, Husain S, Infectious Diseases AST. Community of Practice. Aspergillosis in solid organ transplantation. Am J Transplant 2013;13 Suppl 4:228-41. [Crossref] [PubMed]
- Kawamura S, Maesaki S, Tomono K, et al. Clinical evaluation of 61 patients with pulmonary aspergilloma. Intern Med 2000;39:209-12. [Crossref] [PubMed]
- Prasad A, Agarwal K, Deepak D, et al. Pulmonary Aspergillosis: What CT can Offer Before it is too Late! J Clin Diagn Res 2016;10:TE01-5. [PubMed]
- Ors F, Deniz O, Bozlar U, et al. High-resolution CT findings in patients with pulmonary tuberculosis: correlation with the degree of smear positivity. J Thorac Imaging 2007;22:154-9. [Crossref] [PubMed]
- Kim SH, Kim MY, Hong SI, et al. Invasive Pulmonary Aspergillosis-mimicking Tuberculosis. Clin Infect Dis 2015;61:9-17. [Crossref] [PubMed]
- Hulskotte E, Gupta S, Xuan F, et al. Pharmacokinetic interaction between the hepatitis C virus protease inhibitor boceprevir and cyclosporine and tacrolimus in healthy volunteers. Hepatology 2012;56:1622-30. [Crossref] [PubMed]
- de Jonge H, Vanhove T, de Loor H, et al. Progressive decline in tacrolimus clearance after renal transplantation is partially explained by decreasing CYP3A4 activity and increasing haematocrit. Br J Clin Pharmacol 2015;80:548-59. [Crossref] [PubMed]
- Imamura CK, Furihata K, Okamoto S, et al. Impact of cytochrome P450 2C19 polymorphisms on the pharmacokinetics of tacrolimus when coadministered with voriconazole. J Clin Pharmacol 2016;56:408-13. [Crossref] [PubMed]
- Bhaloo S, Prasad GV. Severe reduction in tacrolimus levels with rifampin despite multiple cytochrome P450 inhibitors: a case report. Transplant Proc 2003;35:2449-51. [Crossref] [PubMed]
- Shih TY, Ho SC, Hsiong CH, et al. Selected pharmaceutical excipient prevent isoniazid and rifampicin induced hepatotoxicity. Curr Drug Metab 2013;14:720-8. [Crossref] [PubMed]
- Meyers BR, Papanicolaou GA, Sheiner P, et al. Tuberculosis in orthotopic liver transplant patients: increased toxicity of recommended agents; cure of disseminated infection with nonconventional regimens. Transplantation 2000;69:64-9. [Crossref] [PubMed]
- Christians U, Jacobsen W, Benet LZ, et al. Mechanisms of clinically relevant drug interactions associated with tacrolimus. Clin Pharmacokinet 2002;41:813-51. [Crossref] [PubMed]
- Ernst A, Gordon FD, Hayek J, et al. Lung abcess complicating Legionella micdadei pneumonia in an adult liver transplant recipient: case report and review. Transplantation 1998;65:130-4. [Crossref] [PubMed]
- Choi YI, Hwang S, Park GC, et al. Clinical outcomes of Pneumocystis carinii pneumonia in adult liver transplant recipients. Transplant Proc 2013;45:3057-60. [Crossref] [PubMed]