Pulmonary tuberculous and tuberculous pericardial tamponade post lung transplant
Introduction
Pulmonary infections are the leading cause of morbidity and mortality among lung transplant recipients (1). Tuberculosis (TB) is a serious opportunistic infection reported in lung transplant recipients. It’s incidence ranges between 6.5% and 10% (2). The incidence of TB among transplanted recipients in North America is 36 to 74-fold higher than in the general population (3).
Lung transplant recipients are at increased risk for infectious complications compared to other solid organ transplant recipients due to the direct contact between the pathogens and the graft in an immunosuppressed patient. Other factors include: the loss of effective lymphatic drainage, and the decrease of mechanical defense due to reduced mucociliary clearance and decreased cough.
US data reports higher incidence of TB infection if organ donors recruited from populations of low social and economic status, infected by the human immunodeficiency virus, and immigrant populations from countries with a high TB (4). The TB could present as pulmonary tuberculosis (2-5), an isolated pleural infection with TB (1,5), this presentation with tuberculous pericardial tamponade after lung transplant is unique.
Case presentation
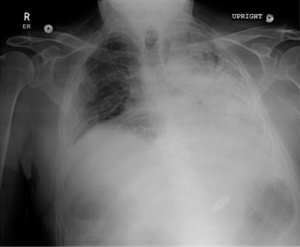
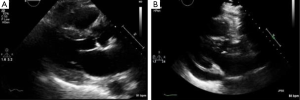
A 73-year-old male with end-stage lung disease secondary to idiopathic pulmonary fibrosis underwent single right lung transplantation, the patient received standard induction immunosuppression protocol with (Cellcept®, Simulect®, and Solu-Medrol®), post-operative course was complicated by prolonged respiratory failure, he was discharged to rehab facility 2 months later with immunosuppression regimen composed of (Cellcept® and Prednisone). He presented to the office 4 months later with shorten of breath (SOB) tiredness and diarrhea, stool culture demonstrated a clostridium difficile infection. He was admitted, and was started on treatment with Metronidazole. A Chest X-ray (CXR) and transthoracic echocardiography (TTE) were stable without new findings. The Bronchoalveolar lavage (BAL) and the sputum cultures were also negative for any infection including TB. During admission, his SOB progressed rapidly, he became lethargic with marginal blood pressure readings, which led to a new CXR (Figure 1) performed 6 days after the admission showed a small right pleural effusion and enlargement of heart silhouette, subsequent TTE revealed a new large pericardial effusion compared to the admission TTE performed 1 week prior (Figure 2A,B). The patient underwent a subxyphoid pericardial window, and a right chest tube insertion. The pericardial fluid chemistry revealed an exudate effusion with glucose of 104 mg/dL, LDH 839 U/L and a ratio of 81%, protein (2.8 g/dL ratio 77%), lymphocytes formed 93% of all fluid nucleated cell, neutrophils formed 3%. Surprisingly the pericardial fluid cultures came back positive for Mycobacterium TB. The sputum culture came back positive repeatedly during the hospital stay. A quadruple anti-TB treatment was started including (RIPE protocol) Rifabutin, Isoniazid, Pyrazinamide, and Ethambutol. The post-operative course was complicated with prolonged respiratory failure, failure to thrive and the family decided to withdraw care 1 month later.
The patient didn’t have a history of TB or recent travelling outside of the USA and his tuberculin skin test was negative. The case was reported to the United Network for Organ Sharing (UNOS) and all recipients of other organs of the same donor were screened for TB after the results of this case were confirmed and surveyed closely and meticulously. Fortunately, no other organ recipient from the same donor showed any signs of TB infection.
Comment
The prevalence of active TB among solid organ transplant recipients in developed countries has ranged from 1.2% to 6.4% (4). The source of TB infection could be difficult to identify. It may be caused by direct exposure to the bacillus after transplantation causing new primary infection, reactivation of a lesion from the donor lung, or reactivation of a non-pulmonary primary infection in the recipient (2,3). Furthermore, the required immunosuppression therapy in transplantation may promote the occurrence of infection (4,6).
The diagnosis of TB in solid organ transplant recipients presents challenges that may lead to treatment delay. These challenges include atypical clinical presentations, increased likelihood of negative tuberculin skin tests and/or interferon-gamma release assays, and negative sputum smear results despite active disease (4,7). The treatment of TB in transplant recipients also has its own challenges, which include pharmacokinetic interactions between immunosuppressive and antituberculous medications, allograft-related drug toxicities, and inadequate immune responses to mycobacterium TB due to exogenous immunosuppression (2,4).
In this case, the diagnosis was made with positive culture of intraoperative sampling of pericardial effusion. We believe that this case presents a new TB infection due to the negative history of TB and traveling outside the US for both the recipient and the donor, no other recipient developed TB, and the intraoperative cultures of donor lung as well as the pathology and the culture of the resected lung were negative. All BAL and sputum cultures after transplant were negative prior to the last admission, a primary pulmonary TB can’t be excluded as most of the time the collected data and performed text on the donor are limited and with the short period before the donation and the harvest procedure, anyhow there is no other TB case reported in other recipients from the same donor.
In summary, pulmonary TB and tuberculous pericarditis is a very rare identity in lung transplant recipients. The clinical manifestations are subtle and the diagnosis is difficult, which might lead to treatment delays, multiple repetition of tests might be needed. TB should be kept in the differential diagnosis in patients presenting with pleural or pericardial effusion and a high level of lymphocyte concentration in fluid analysis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Fairhurst RM, Kubak BM, Shpiner RB, et al. Mycobacterium abscessus empyema in a lung transplant recipient. J Heart Lung Transplant 2002;21:391-4. [Crossref] [PubMed]
- Morales P, Briones A, Torres JJ, et al. Pulmonary tuberculosis in lung and heart-lung transplantation: fifteen years of experience in a single center in Spain. Transplant Proc 2005;37:4050. [Crossref] [PubMed]
- Torre-Cisneros J, Doblas A, Aguado JM, et al. Tuberculosis after solid-organ transplant: incidence, risk factors, and clinical characteristics in the RESITRA (Spanish Network of Infection in Transplantation) cohort. Clin Infect Dis 2009;48:1657. [Crossref] [PubMed]
- Singh N, Paterson DL. Mycobacterium tuberculosis infection in solid-organ transplant recipients: impact and implications for management. Clin Infect Dis 1998;27:1266. [Crossref] [PubMed]
- Wahidi MM, Willner DA, Snyder LD, et al. Diagnosis and Outcome of Early Pleural Space Infection Following Lung Transplantation. Chest 2009;135:484-91. [Crossref] [PubMed]
- Baumann MH, Nolan R, Petrini M, et al. Pleural tuberculosis in the United States: incidence and drug resistance. Chest 2007;131:1125-32. [Crossref] [PubMed]
- Studer SM, Levy RD, McNeil K, et al. Lung transplant outcomes: a review of survival, graft function, physiology, health-related quality of life and cost-effectiveness. Eur Respir J 2004;24:674-85. [Crossref] [PubMed]