Epigenetics of colorectal cancer: emerging circulating diagnostic and prognostic biomarkers
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related death in Western countries.
Despite consistent improvements in screening strategies and the development of more effective treatments, the 5-year survival rates for advanced cancer is still unpromising (1).
Classically, CRC has been considered a complex disease that arises as result of the accumulation of genetic alterations in key regulatory genes and pathways, including the RAS-MAPK pathway with KRAS, NRAS, and BRAF genes, Wnt and P13K pathways (2). Subsequently, it became clear that genetic mutations play, in fact, only a partial role in colorectal carcinogenesis. Epigenetic variations in cancer-related genes and noncoding RNAs are now believed to be strongly involved in cancer initiation and progression. They occur widely across the genome and constitute an important cause of tumour heterogeneity. Nowadays, multiple ways for epigenetic modification are known to play pivotal role in CRC including microsatellite instability, histone modifications, DNA methylation, chromatin remodelers and non-coding RNAs (3). Consequently, epigenetic alterations represent an attractive target either for epidemiological and physiopathological studies or for therapeutic response evaluation and drug design (4-6). Furthermore, they have been recently proposed as new diagnostic and prognostic biomarkers. In the present review, we will provide an overview of the clinical applications of the most extensively studied epigenetic alterations as diagnostic and prognostic biomarkers for CRC. In particular we focused on DNA methylation and microRNAs (miRNAs) which are the epigenetic biomarkers currently most studied in CRC.
DNA methylation
DNA methylation consists to the enzymatic addition of a methyl group to cytosine in 5-position. The process is catalyzed by DNA methyltransferases and usually entails a covalent linkage within a CG dinucleotide sequence, termed CpG transcription (7). In normal mammalian cells CpGs can be found as single dinucleotides spread throughout the genome or concentrated in large clusters conventionally known as “CpG islands” representing sequences of 200–500 bases in length with greater than 50% GC content. In normal mammalian cells, the majority of the CpG sites are heavily methylated while CpG islands, usually located in the promoter regions of genes, are unmethylated. Interestingly, during cancer initiation, hyper-methylation within the promoter region may lead to inactivation of tumor-suppressor genes, while generalized hypo-methylation is associated with genomic instability and chromosomal aberrations (8).
Global DNA hypomethylation
The global loss of DNA methylation predominantly affects CpG dinucleotides found in repetitive sequences of DNA, such as Long Interspersed Nucleotide Element 1 (LINE-1) and Alu repeats.
LINE-1 repeats are distributed throughout the genome and occupy approximately 18% of the genome. LINE-1 has retrotransposition activity, an ability through which, upon hypomethylation, LINE-1 can copy itself and mobilize new copies to novel genomic locations. Line-1, as retrotransposon, heavily impacts the structure of the genome and may have adverse effects on genome stability thus contributing to cancer initiation and progression (9,10). To date LINE-1 methylation levels have been intensively studied in almost all human cancer types and used as surrogate biomarkers to estimate the DNA methylation level across the genome in cancer tissues and in white blood cells. Recently, a meta-analysis including 19 unique articles published between 2004 and 2014 has been performed in order to evaluate the association between blood and tissue LINE-1 hypomethylation and cancer risk (11). The quantitative analysis, performed on 2,554 cancer patients and 3,553 healthy controls, demonstrated that the mean methylation level in cancer patients was 6.4% lower than in healthy controls. A subgroup analysis by specific cancer types revealed that LINE-1 hypomethylation increases at a statistically significant level in colorectal and gastric cancer but not in hepatocellular carcinoma (HCC). In particular, in the subgroup analysis on CRC studies, including 693 patients and 481 controls, the LINE-1 methylation levels were 8.3% lower in patients than in controls (95% CI: −10.56 to −6.10, P<0.001).
Interesting, studies on colorectal and gastric cancer investigated LINE-1 methylation in tissue speciments, while included studies on HCC evaluated the association in peripheral leukocytes. Accordingly, the meta-analysis strongly confirmed that an association between LINE-1 hypomethylation and increased cancer risk exist but is proven only in tissue samples both fresh/frozen and formalin-fixed paraffin-embedded (FFPE) tissues and not in blood samples. In another well powered cross-sectional study, King and coauthors reported a statistically significant inverse relationship between LINE-1 methylation in colon tissue and adenoma risk for the lowest methylation quartile compared to the highest (adjusted OR: 2.26, 95% CI: 1.11–4.58, P=0.02) (12). The same analysis performed on blood failed to reveal a statistically significant association although the overall pattern of odds ratio for increased risk showed a trend across increasing methylation quartiles (adjusted OR: 1.39, 95% CI: 0.67–2.89, P=0.37). A weak correlation was found between blood and tissue methylation levels (r =0.36).
These finding were in concordance with previous evidences demonstrating that, in bladder and colon cancer, the global hypomethylation detected in tumor tissues was not found in blood samples (13,14).
On the contrary, Walters et al. (15) reported strong evidence of an association between high levels of LINE-1 methylation in white blood cells and increased risk of CRC (OR: 2.34, 95% CI: 1.48–3.70, P<0.001).
To date, many studies have also evaluated the association between LINE-1 hypomethylation and clinical outcome. Overall, studies on tissue LINE-1 methylation consistently demonstrated that LINE-1 hypomethylation was associated with higher CRC-specific mortality especially in early stage cancer (16-18) while evidence coming from studies on circulating LINE-1 are still lacking.
Promoter hypermethylation
Diagnostic role of circulating biomarkers
Aberrant gene promoter methylation in the plasma or serum of patients with CRC has been shown great promise as potential diagnostic indicator of CRC. To date, a lot of hypermethylated genes have been reported in CRC, but only few have been included in commercial blood-based test.
SEPT9 is one of the most widely studied genes. The first works to evaluate methylated SEPT9 in the plasma of CRC patients were published in 2008 (19,20). In these preliminary reports on research kits the SEPT9 assay already exhibited high sensitivity and specificity for CRC detection. After the improvement of the method and subsequent availability of the first generation commercialized product (Epi proColon 1.0, Epigenomics AG, Berlin) and, subsequently of the second-generation one (Epi proColon 2.0), the detection sensitivity increased from about 60–70% to about 70–90%, while the specificity increased from 80–90% to above 90% (21). Notably, second-generation Epi proColon SEPT9 assay exhibited a better sensitivity and almost equal specificity to that of the most commonly used standard guaiac-based fecal occult blood test (gFOBT) (22). More specifically, the second generation SEPT9 assay showed a clear advantage over gFOBT test in detecting right-sided CRCs with sensitivities of 94.4% and 50% respectively. With respect to the first generation assay, the new kit implies fewer handling steps, fewer reagents and a reduced overall processing time. More importantly, the procedure includes three independent PCR replicates instead of two, which explains the enhanced sensitivity. However, methylated SEPT9 of both first and second generation have shown a limited sensitivity for the detection of advanced adenomas (27.4%), underscoring the need for further improvement of this test in population-based screening setting (23).
Since combining multiple biomarkers has become a trend in CRC detection and screening, several authors evaluated the diagnostic performance of SEPT9 assay along with other blood-based candidate methylated genes. The association of SEPT9 with TAC1 methylation assay yielded a sensitivity of 73.1% and a specificity of 92.3% (24) while its association with TMEFF2 and ALX4, further increased both sensitivity (80.7%) and specificity (90.0%) (25). More importantly, Tanzer and coauthors recently demonstrated that the combination of SEPT9 with ALX4 led to a sensitivity of 71% and a specificity of 95% for advanced adenomas, thus supporting SEPT9/ALX4 as a biomarker for precancerous lesions (26).
Apart from SEPT9 the role of methylation in CRC detection has been investigated for a number of genes including HJC1, CYCD2, PAX5, RB1, SRBC, NPY, PENK, WIF1, ALX4, HLFT, HPP1, MLH1, APC, CDKN2A/P16h, TMEFF2, NGFR, FRP2, NEUROG1, and RUNX3. Studies evaluating serum or plasma methylated biomarkers different from SEPT9 showed modest diagnostic performance with sensitivities between 33% (SRBC) and 97% (CYCD2) and specificities between 37% (CYCD2) and 100% [helicase-like transcription factor (HLTF), HPP1 and MLH1], no one of the candidates showing a good combination of sensitivity and specificity (27).
Recently, Li and coauthors performed a systematic review and meta-analysis to establish the sensitivity and specificity of hypermethylated blood-based biomarkers for CRC detection. After an initial search including 981 articles, 39 were finally included in the meta-analysis accounting for a total of 3,853 patients and 6,431 controls. Single and combined genes were targeted, and serum or plasma samples from patients with early and advanced stages were used. Data from subgroup analysis showed that the pooled sensitivity was higher for studies evaluating SEPT9 methylation than for studies evaluating genes different from SEPT9 (75% vs. 58%) while the pooled specificity was almost comparable (0.89 vs. 0.91). No significant difference among other subgroups including multiple target genes vs. single target gene and qPCR based methods vs. other methods were observed (28).
Prognostic role of circulating biomarkers
A small number of circulating methylated genes appear to have diagnostic potential for CRC, but far fewer exhibit convincing prognostic relevance. To date, the most studied candidate biomarkers are the methylation status of p16, HLTF and O6-Methylguanine-DNA-methyltransferase (MGMT) (29-31). In view of its prominent role as tumour suppressor, the silencing of p16, subsequent to its promoter methylation, has been suggested to contribute to CRC carcinogenesis. Accordingly, many studies have tried to assess whether the p16 promoter hypermethylation may represent a prognostic marker for CRC. Some investigations reported a correlation between p16 promoter methylation and poorer survival in patients with CRC while others, despite having observed a trend of positive effect, found it not statistically significant. In order to quantitatively analyze the association between p16 hepermethylation and prognosis in CRC patients Jiang and coauthors performed a meta-analysis on the overall and disease free survival including 16 studies for a total of 3,968 patients for the first end-point and six studies for a total of 1,091 patients for the second end-point. The meta-analysis demonstrated that p16 hypermethylation is significantly associated with both poor overall and disease free survival (HR: 1.64, 95% CI: 1.3–2.0 and HR: 1.9, 95% CI: 1.2–3.2 respectively) thus suggesting a decisive confirmation of the adverse prognostic effect of p16 promoter methylation in CRC (32).
The methylation of HLTF in cfDNA has been shown to strongly correlate with tumor size, lymph node metastasis, histological grading and tumor stage. It was also initially found to be associated with an increased risk of disease recurrence (RR: 2.5, 95% CI: 1.1–5.6, P=0.023) (33) and with increased risk of death (RR: 3.4, 95% CI: 1.4–8.1; P=0.007) (30). However, a later validation study by same authors failed to replicate such prognostic significance (34).
MGMT encodes the DNA-repair protein O-alkylguanine (O6-AG) DNA alkyl-transferase (AGT) which can remove the natural occurring toxic lesions from O6-guanine in DNA. MGMT protect cells against these lesions, transferring the alkyl group to one of the internal cysteine residues on each repair protein. One MGMT molecule is inactivated for each lesion that is repaired (35). Accordingly, epigenetic silencing of MGMT which occurs as a consequence of promoter methylation creates a preconditioned genetic field for colorectal carcinogenesis and may thus represent a predisposing factor for cancer development. Between 2003 and 2010, five studies investigated the prognostic role of MGMT promoter methylation in CRC but with inconsistent results due to limited statistical power (n<200) (36-40). Finally, in 2011, analyzing a database of 855 CRC in two large prospective studies, Shima and coauthors concluded that MGMT alteration was not associated with patient prognosis in CRC (41).
Recently, Liu and colleagues performed a prospective study (42), involving 165 CRC patients, to explore the prognostic potential of seven candidate methylated genes selected for having resulted more methylated in CRC patients as compared to healthy controls (24). Such genes were: T-cell differentiation protein (MAL), SEPT9, tachykinin-1 (TAC1), nel-like type 1 (NELL1), cellular retinoic acid binding protein 1 (CRABP1), somatostatin (SST) and eyes absent homolog 4 (EYA4). In univariate analysis, cancer-specific survival was significantly influenced not only by traditional clinic-pathological parameters and serum CEA, but also by serum methylation levels of MAL and SST. In multivariate Cox analysis adjusted for all other significant factors, serum methylated SST (mSST) remained a significant and independent predictor of poor prognosis. Patients with high serum mSST were noted to have a higher risk of cancer-related mortality (HR: 1.96, 95% CI: 1.06–3.62, P=0.031), higher risk of CRC recurrence (HR: 2.60, 95% CI: 1.37–4.94, P=0.003) compared to patients with low methylation levels. Such results were confirmed in a subsequent study performed by the same authors in an independent cohort of 150 CRC patients (43). Notably, SST, encoding a well-characterized gastrointestinal neuroendocrine and growth-regulatory peptide, acts as a tumor suppressor gene and possesses potent antitumor abilities. It exerts antitumor effects by multiple mechanisms including the induction of cell cycle arrest and apoptosis, the control of cell proliferation and the inhibition of cell invasion. The epigenetic involvement of SST gene silencing in CRC is supported by observations that SST methylation levels in tumor tissues are significantly higher than that in adjacent normal mucosa of CRC patients or normal mucosa from healthy controls (44) and that hypermethylation was found in percentages ranging from 88% to 100% in primary tumours (42,44).
miRNAs
miRNAs are short non-coding RNA fragments of 19–22 nucleotides in length involved in post-transcriptional regulation of gene expression. In physiological conditions, miRNAs can regulate a hundreds of biological pathways which among the others include cell differentiation, proliferation and survival. Altered miRNA expression has been associated with known disease processes or conditions including cancer. By targeting either oncogenes or tumor suppressor genes, miRNAs have shown to play an important role in the multistep processes of carcinogenesis and have hence be proposed as new potential diagnostic prognostic and predictive biomarkers in cancer research. Interesting, miRNAs can be actively released from cancer cells and can been found in detectable levels in body fluids (45,46). Moreover, circulating miRNAs are remarkably stable and consistent among individuals. Accordingly, tumor derived miRNAs in serum or plasma emerged as potentially non-invasive and reliable new cancer diagnostic biomarkers (47).
A number of studies have demonstrated that some miRNAs are abnormally expressed in CRC plasma or serum samples. Unfortunately, due to the lack of standardized protocols for miRNAs extraction, normalization and quantification, available results in literature remain still poor reproducible.
Thus, in the follow paragraph we reported the results from studies that assessed the diagnostic and prognostic role of different miRNAs in CRC by selecting candidates in a screening phase and by confirming their potential in a validation phase. Most promising miRNAs have been summarized in Tables 1,2.
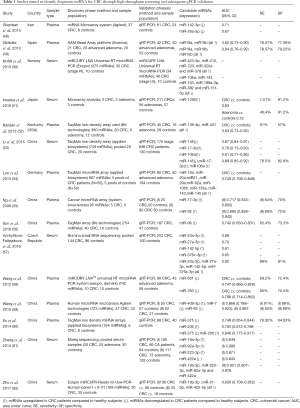
Full table
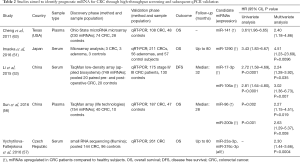
Full table
Diagnostic role of circulating miRNAs
The role of circulating miRNAs as biomarkers for the diagnosis of CRC has been recently assessed by Carter and coauthors who performed a careful systematic review and meta-analysis of studies evaluating plasma or serum miRNAs in the diagnosis of CRC (64). The literature search was performed in the timeframe between January 2002 and April 2016 and finally included 19 articles for a total of 6,010 patients (3,454 CRC patients and 2,556 healthy controls). Twelve (48-50,52-56,58-61) out of these 19 articles used high throughput technologies to identify candidate miRNAs in a discovery cohort and then qRT-PCR to validate the performance of selected miRNAs in at least one independent validation cohort. The main characteristics and results of these studies are reported in Table 1 along with those of three further article structured in a similar manner and published after the meta-analysis (51,57,62). Notably three upregulated miRNAs, miR-19a-3p, miR-21 and miR-92, were identified as promising diagnostic biomarker by more than one study and always with AUC higher than 0.80. Moreover, although numerous single miRNAs showed to distinguish patients with CRC from healthy controls with high sensitivity and specificity, the combination of most dysregulated miRNAs into a panel usually reached better diagnostic performance.
Prognostic role of circulating miRNAs
Since it has been demonstrated that several miRNAs are closely correlated with CRC cell proliferation, invasion, lymph node metastases and advanced clinical stage, a number of studies investigated the role of most dysregulated miRNAs as prognostic biomarkers for CRC survival.
One of most extensively investigated miRNA is miR-21 whom tissue expression was reported to be independently associated with poor prognosis by two meta-analyses (65,66). The first study demonstrating that high levels of serum (rather than tissue) miR-21 indicate a poor prognosis in patients with CRC, was published in 2013 by Toiyama and coauthors (67). In a large validation cohort of 186 CRC patients, serum miR-21 expression levels were found to be associated with tumor size, distant metastasis and advanced TNM stage. Importantly, the authors demonstrated that serum miR-21 levels significantly correlated with matched tissue expression levels (r=0.32; 95% CI: 0.17–0.45; P<0.001) thus supporting its use as non-invasive biomarker. Moreover, contrarily to tissue miR-21, whose expression in tumours was statistically significantly compromised by other clinical factors including pathological stage and grade, serum miR-21 was an independent prognostic factor for overall survival (HR: 4.12, 95% CI: 1.10–15.4; P=0.03) (67).
Most relevant studies aimed to identify circulating miRNAs as clinical outcome predictors by using a two-step approach as for studies on diagnostic miRNAs are summarized in Table 2 (51,53,56,57,63). Each of the five studies summarized found each own best candidate with no reproducible results.
Conclusions
Considering the substantial role of epigenetic alteration in CRC initiation and progression, extensive research has been undertaken over the last decade to identify new biomarkers for CRC. The assessment of epigenetic biomarkers in serum or plasma has shown the potential to become a viable non-invasive alternative to the current, poor effective, screening strategy, especially for those tumors such as CRC characterized by poor adherence rate. Nevertheless, our understanding of CRC epigenetics in blood is still far from being elucidated and both biological and methodological challenges need further evaluation (68,69).
Aberrant DNA methylation and dysregulation of miRNAs expression have been the most studied traits of epigenetic alteration in CRC, and it is widely recognised that global hypomethylation, promoter hypermethylation as well as miRNAs alterations can have a role in CRC diagnosis and prognostication. Continued investigation of these promising classes of biomarkers promises to lead to a high performance tool that can be used to prevent and manage patients with CRC. At present, however, only few studies have been conducted with methodological rigor as to ensure the reliability of results while most of literature comprises initial exploratory studies that suffered from methodologic weaknesses, including small sample size, non-clear patient information, lack of replication, and poor statistical analysis (70-72).
To date a general consensus has been reached only for one application which is the use of SEPT9 methylation assay for the early diagnosis of CRC but encouraging results have also been found for some diagnostic miRNAs including miR-19a-3p, miR-21 and miR-92. Further studies on the associated mechanisms behind miRNAs dysregulations and DNA methylation are needed to identify new reliable prognostic biomarkers to be tested in large validation studies. From the data presented here it is reasonable to expect that a multi-markers approach might provide a better tool than a single biomarker in both diagnosis and prognostication of CRC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clin Colon Rectal Surg 2009;22:191-7. [Crossref] [PubMed]
- Cunningham D, Atkin W, Lenz HJ, et al. Colorectal cancer. Lancet 2010;375:1030-47. [Crossref] [PubMed]
- Lao VV, Grady WM. Epigenetics and colorectal cancer. Nat Rev Gastroenterol Hepatol 2011;8:686-700. [Crossref] [PubMed]
- Pancione M, Remo A, Colantuoni V. Genetic and epigenetic events generate multiple pathways in colorectal cancer progression. Patholog Res Int 2012;2012:509348. [Crossref] [PubMed]
- Bardhan K, Liu K. Epigenetics and colorectal cancer pathogenesis. Cancers 2013;5:676-713. [Crossref] [PubMed]
- Barrow TM, Michels KB. Epigenetic epidemiology of cancer. Biochem Biophys Res Commun 2014;455:70-83. [Crossref] [PubMed]
- Kulis M, Esteller M. DNA methylation and cancer. Adv Genet 2010;70:27-56. [Crossref] [PubMed]
- Siegfried Z, Simon I. DNA methylation and gene expression. Wiley Interdisciplinary Reviews: Systems Biology and Medicine 2010;2:362-71. [Crossref] [PubMed]
- Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature 2001;409:860-921. [Crossref] [PubMed]
- Deininger PL, Moran JV, Batzer MA, et al. Mobile elements and mammalian genome evolution. Curr Opin Genet Dev 2003;13:651-8. [Crossref] [PubMed]
- Barchitta M, Quattrocchi A, Maugeri A, et al. LINE-1 hypomethylation in blood and tissue samples as an epigenetic marker for cancer risk: a systematic review and meta-analysis. PLoS One 2014;9:e109478. [Crossref] [PubMed]
- King WD, Ashbury JE, Taylor SA, et al. A cross-sectional study of global DNA methylation and risk of colorectal adenoma. BMC Cancer 2014;14:488. [Crossref] [PubMed]
- Wolff EM, Byun HM, Han HF, et al. Hypomethylation of a LINE-1 Promoter Activates an Alternate Transcript of the MET Oncogene in Bladders with Cancer. PLoS Genet 2010;6:e1000917. [Crossref] [PubMed]
- Suter CM, Martin DI, Ward RL. Hypomethylation of L1 retrotransposons in colorectal cancer and adjacent normal tissue. Int J Colorectal Dis 2004;19:95-101. [Crossref] [PubMed]
- Walters RJ, Williamson EJ, English DR, et al. Association between hypermethylation of DNA repetitive elements in white blood cell DNA and early-onset colorectal cancer. Epigenetics 2013;8:748-55. [Crossref] [PubMed]
- Benard A, van de Velde CJ, Lessard L, et al. Epigenetic status of LINE-1 predicts clinical outcome in early-stage rectal cancer. Br J Cancer 2013;109:3073-83. [Crossref] [PubMed]
- Swets M, Zaalberg A, Boot A, et al. Tumor LINE-1 Methylation Level in Association with Survival of Patients with Stage II Colon Cancer. Int J Mol Sci 2016;18:E36. [Crossref] [PubMed]
- Mima K, Nowak JA, Qian ZR, et al. Tumor LINE-1 methylation level and colorectal cancer location in relation to patient survival. Oncotarget 2016;7:55098-109. [PubMed]
- Grützmann R, Molnar B, Pilarsky C, et al. Sensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assay. PLoS One 2008;3:e3759. [Crossref] [PubMed]
- Lofton-Day C, Model F, Devos T, et al. DNA methylation biomarkers for blood-based colorectal cancer screening. Clin Chem 2008;54:414-23. [Crossref] [PubMed]
- Song L, Li Y. SEPT9: A Specific Circulating Biomarker for Colorectal Cancer. Adv Clin Chem 2015;72:171-204. [Crossref] [PubMed]
- Tóth K, Sipos F, Kalmár A, et al. Detection of methylated SEPT9 in plasma is a reliable screening method for both left- and right-sided colon cancers. PLoS One 2012;7:e46000. [Crossref] [PubMed]
- Jin P, Kang Q, Wang X, et al. Performance of a second-generation methylated SEPT9 test in detecting colorectal neoplasm. J Gastroenterol Hepatol 2015;30:830-3. [Crossref] [PubMed]
- Liu Y, Tham CK, Ong SY, et al. Serum methylation levels of TAC1 SEPT9 and EYA4 as diagnostic markers for early colorectal cancers: a pilot study. Biomarkers 2013;18:399-405. [Crossref] [PubMed]
- He Q, Chen HY, Bai EQ, et al. Development of a multiplex MethyLight assay for the detection of multigene methylation in human colorectal cancer. Cancer Genet Cytogenet 2010;202:1-10. [Crossref] [PubMed]
- Tänzer M, Balluff B, Distler J, et al. Performance of epigenetic markers SEPT9 and ALX4 in plasma for detection of colorectal precancerous lesions. PLoS One 2010;5:e9061. [Crossref] [PubMed]
- Lam K, Pan K, Linnekamp JF, et al. DNA methylation based biomarkers in colorectal cancer: A systematic review. Biochim Biophys Acta 2016;1866:106-20. [PubMed]
- Li B, Gan A, Chen X, et al. Diagnostic Performance of DNA Hypermethylation Markers in Peripheral Blood for the Detection of Colorectal Cancer: A Meta-Analysis and Systematic Review. PLoS One 2016;11:e0155095. [Crossref] [PubMed]
- Nakayama G, Hibi K, Kodera Y, et al. P16 methylation in serum as a potential marker for the malignancy of colorectal carcinoma. Anticancer Res 2007;27:3367-70. [PubMed]
- Wallner M, Herbst A, Behrens A, et al. Methylation of serum DNA is an independent prognostic marker in colorectal cancer. Clin Cancer Res 2006;12:7347-52. [Crossref] [PubMed]
- Bazan V, Bruno L, Augello C, et al. Gruppo Oncologico dell’Italia Meridionale. Molecular detection of TP53, Ki-Ras and p16INK4A promoter methylation in plasma of patients with colorectal cancer and its association with prognosis. Results of a 3-year GOIM (GruppoOncologicodell’ItaliaMeridionale) prospective study. Ann Oncol 2006;17:vii84-90. [Crossref] [PubMed]
- Jiang W, Wang PG, Zhan Y, et al. Prognostic value of p16 promoter hypermethylation in colorectal cancer: a meta-analysis. Cancer Invest 2014;32:43-52. [Crossref] [PubMed]
- Herbst A, Waller M, Rahmig K, et al. Methylation of helicase-like transcription factor in serum of patients with colorectal cancer is an independent predictor of disease recurrence. Eur J Gastroenterol Hepatol 2009;21:565-9. [Crossref] [PubMed]
- Philipp AB, Stiber P, Nagel D, et al. Prognostic role of methylated free circulating DNA in colorectal cancer. Int J Cancer 2012;131:2308-19. [Crossref] [PubMed]
- Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer 2004;4:296-307. [Crossref] [PubMed]
- Nagasaka T, Sharp GB, Notohara K, et al. Hypermethylation of O6-methylguanine-DNA methyltransferase promoter may predict nonrecurrence after chemotherapy in colorectal cancer cases. Clin Cancer Res 2003;9:5306-12. [PubMed]
- Krtolica K, Krajnovic M, Usaj-Knezevic S, et al. Comethylation of p16 and MGMT genes in colorectal carcinoma: correlation with clinicopathological features and prognostic value. World J Gastroenterol 2007;13:1187-94. [Crossref] [PubMed]
- Chen SP, Chiu SC, Wu CC, et al. The association of methylation in the promoter of APC and MGMT and the prognosis of Taiwanese CRC patients. Genet Test Mol Biomarkers 2009;13:67-71. [Crossref] [PubMed]
- Kim JC, Choi JS, Roh SA, et al. Promoter methylation of specific genes is associated with the phenotype and progression of colorectal adenocarcinomas. Ann Surg Oncol 2010;17:1767-76. [Crossref] [PubMed]
- Kohonen-Corish MR, Daniel JJ, Chan C, et al. Low microsatellite instability is associated with poor prognosis in stage C colon cancer. J Clin Oncol 2005;23:2318-24. [Crossref] [PubMed]
- Shima K, Morikawa T, Baba Y, et al. MGMT promoter methylation, loss of expression and prognosis in 855 colorectal cancers. Cancer Causes Control 2011;22:301-9. [Crossref] [PubMed]
- Liu Y, Chew MH, Tham CK, et al. Methylation of serum SST gene is an independent prognostic marker in colorectal cancer. Am J Cancer Res 2016;6:2098-108. [PubMed]
- Tham C, Chew M, Soong R, et al. Postoperative serum methylation levels of TAC1 and SEPT9 are independent predictors of recurrence and survival of patients with colorectal cancer Cancer 2014;120:3131-41. [Crossref] [PubMed]
- Mori Y, Cai K, Cheng Y, et al. A genome-wide search identifies epigenetic silencing of somatostatin, tachykinin-1, and 5 other genes in colon cancer. Gastroenterology 2006;131:797-808. [Crossref] [PubMed]
- Weber JA, Baxter DH, Zhang S, et al. The microRNA spectrum in 12 body fluids. Clin Chem 2010;56:1733-41. [Crossref] [PubMed]
- Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008;105:10513-8. [Crossref] [PubMed]
- Kawaguchi T, Komatsu S, Ichikawa D, et al. Circulating MicroRNAs: A Next-Generation Clinical Biomarker for Digestive System Cancers. Int J Mol Sci 2016;17:E1459. [Crossref] [PubMed]
- Ghanbari R, Mosakhani N, Asadi J, et al. Downregulation of Plasma MiR-142-3p and MiR-26a-5p in Patients With Colorectal Carcinoma. Iran J Cancer Prev 2015;8:e2329. [Crossref] [PubMed]
- Giráldez MD, Lozano JJ, Ramírez G, et al. Circulating microRNAs as biomarkers of colorectal cancer: results from a genome-wide profiling and validation study. Clin Gastroenterol Hepatol 2013;11:681-8.e3. [Crossref] [PubMed]
- Hofsli E, Sjursen W, Prestvik WS, et al. Identification of serum microRNA profiles in colon cancer. Br J Cancer 2013;108:1712-9. [Crossref] [PubMed]
- Imaoka H, Toiyama Y, Fujikawa H, et al. Circulating microRNA-1290 as a novel diagnostic and prognostic biomarker in human colorectal cancer. Ann Oncol 2016;27:1879-86. [Crossref] [PubMed]
- Kanaan Z, Roberts H, Eichenberger MR, et al. A plasma microRNA panel for detection of colorectal adenomas: a step toward more precise screening for colorectal cancer. Ann Surg 2013;258:400-8. [Crossref] [PubMed]
- Li J, Liu Y, Wang C, et al. Serum miRNA expression profile as a prognostic biomarker of stage II/III colorectal adenocarcinoma. Sci Rep 2015;5:12921. [Crossref] [PubMed]
- Luo X, Stock C, Burwinkel B, et al. Identification and evaluation of plasma microRNAs for early detection of colorectal cancer. PLoS One 2013;8:e62880. [Crossref] [PubMed]
- Ng EK, Chong WW, Jin H, et al. Differential expression of microRNAs in plasma of patients with colorectal cancer: a potential marker for colorectal cancer screening. Gut 2009;58:1375-81. [Crossref] [PubMed]
- Sun Y, Liu Y, Cogdell D, et al. Examining plasma microRNA markers for colorectal cancer at different stages. Oncotarget 2016;7:11434-49. [PubMed]
- Vychytilova-Faltejskova P, Radova L, Sachlova M, et al. Serum-based microRNA signatures in early diagnosis and prognosis prediction of colon cancer. Carcinogenesis 2016;37:941-50. [Crossref] [PubMed]
- Wang Q, Huang Z, Ni S, et al. Plasma miR-601 and miR-760 are novel biomarkers for the early detection of colorectal cancer. PLoS One 2012;7:e44398. [Crossref] [PubMed]
- Wang S, Xiang J, Li Z, et al. A plasma microRNA panel for early detection of colorectal cancer. Int J Cancer 2015;136:152-61. [Crossref] [PubMed]
- Xu L, Li M, Wang M, et al. The expression of microRNA-375 in plasma and tissue is matched in human colorectal cancer. BMC Cancer 2014;14:714. [Crossref] [PubMed]
- Zheng G, Du L, Yang X, et al. Serum microRNA panel as biomarkers for early diagnosis of colorectal adenocarcinoma. Br J Cancer 2014;111:1985-92. [Crossref] [PubMed]
- Zhu M, Huang Z, Zhu D, et al. A panel of microRNA signature in serum for colorectal cancer diagnosis. Oncotarget 2017;8:17081-91. [PubMed]
- Cheng H, Zhang L, Cogdell DE, et al. Circulating plasma MiR-141 is a novel biomarker for metastatic colon cancer and predicts poor prognosis. PLoS One 2011;6:e17745. [Crossref] [PubMed]
- Carter JV, Galbraith NJ, Yang D, et al. Blood-based microRNAs as biomarkers for the diagnosis of colorectal cancer: a systematic review and meta-analysis. Br J Cancer 2017;116:762-74. [Crossref] [PubMed]
- Zhang H, Li P, Ju H, et al. Diagnostic and prognostic value of microRNA-21 in colorectal cancer: an original study and individual participant data meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:2783-92. [Crossref] [PubMed]
- Xia X, Yang B, Zhai X, et al. Prognostic role of microRNA-21 in colorectal cancer: a meta-analysis. PLoS One 2013;8:e80426. [Crossref] [PubMed]
- Toiyama Y, Takahashi M, Hur K, et al. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer. J Natl Cancer Inst 2013;105:849-59. [Crossref] [PubMed]
- Danese E, Minicozzi AM, Benati M, et al. Comparison of genetic and epigenetic alterations of primary tumors and matched plasma samples in patients with colorectal cancer. PLoS One 2015;10:e0126417. [Crossref] [PubMed]
- Danese E, Minicozzi AM, Benati M, et al. Epigenetic alteration: new insights moving from tissue to plasma - the example of PCDH10 promoter methylation in colorectal cancer. Br J Cancer 2013;109:807-13. [Crossref] [PubMed]
- Moldovan L, Batte KE, Trgovcich J, et al. Methodological challenges in utilizing miRNAs as circulating biomarkers. J Cell Mol Med 2014;18:371-90. [Crossref] [PubMed]
- Kurdyukov S, Bullock M. DNA Methylation Analysis: Choosing the Right Method. Biology (Basel) 2016;5:E3. [Crossref] [PubMed]
- Laird PW. Principles and challenges of genomewide DNA methylation analysis. Nat Rev Genet 2010;11:191-203. [Crossref] [PubMed]


