The role of reactive oxygen species in myocardial redox signaling and regulation
Introduction
Heart failure has emerged as a true epidemic in the western world during the past two decades by appearing most commonly in patients with coronary artery disease. Increasing evidence suggest that oxidative stress (OS) is involved in the pathogenesis of ischemic heart disease, cardiac hypertrophy and congestive heart failure (1,2).
The deleterious effects of oxidants are partially due to the ability of these metabolites to produce modifications in subcellular organelles such as sarcolemma, sarcoplasmic reticulum (SR), mitochondria and nucleus, which are intimately involved in the regulation of cardiomyocyte Ca2+ homeostasis (1,2). OS plays a major role in regulating a wide variety of cellular functions, including gene expression, cell growth, and death. Reactive oxygen species (ROS) post-translationally modulate signaling molecules and transcription factors. ROS generation is efficiently stabilized by antioxidant enzymatic activity that scavenges ROS and limits toxicity. Of most importance are copper-zinc (CuZn) and manganese (Mn) superoxide dismutase (SOD) that dismutate O2– to H2O2, and glutathione peroxidase (GPX) and catalase (CAT), which convert H2O2 to water (3). In conclusion, OS is the combined result of increased ROS production, arising from enzymatic stimulation, including xanthine oxidoreductase (XOR), cyclooxygenase, nitric oxide synthase (NOS), and NADPH oxidases (Noxs), and impaired antioxidant capacity, such as decreased activity of Cu/Zn SOD and CAT.
Sources of ROS in human myocardium
The major potential sources of ROS in human myocardium include the mitochondrial electron transport chain, XOR, NADPH oxidases as well as dysfunctional NOSs.
Excessive ROS production from mitochondria, more specifically from mitochondrial oxidases, has been described in experimental models of myocardial infarction (MI) and heart failure (4,5). Increased production of ROS in the failing heart leads to increased mitochondrial permeability, which causes matrix swelling, outer membrane rupture, the release of apoptotic signaling molecules, such as cytochrome C from the intermembrane space, and irreversible injury of the mitochondria (6). Normally, mitochondrial electron transport chain generates ATP transferring electrons from nicotinamide adenine dinucleotide (NADH) and reduced flavine-adenine dinucleotide (FADH2) to oxygen, reducing it to water. Mitochondrial electron transport chain, localized in the inner mitochondrial membrane, is consisted of four cytochrome-based enzymatic complexes (complexes I, II, III, IV). Complexes I and II are mainly formed by dehydrogenases, while complex III and IV are formed by cytochrome oxidases. Moreover, coenzyme Q transfers electrons between complexes I or II and III and between complexes III and IV. Normally, there is a small percentage 1–2% of electrons “leak” generating ROS, which is scavenged by mitochondrial antioxidant enzymes. However, in pathological conditions, coenzyme Q turns into a primary source of O2– as some electrons may be swerved at the level of complex I or III (7).
Xanthine oxidoreductase represents another major source of ROS in the human heart and is upregulated in clinical states such as HF. It exists in two types xanthine dehydrogenase (XDH) and xanthine oxidase (XO) (8). Both XDH and XO catalyze the conversion of hypoxanthine to xanthine and xanthine to uric acid. More specifically XO, which exclusively reduces oxygen compared to XDH, constitutes a major source of O2– and H2O2 radicals. It has also been described that H2O2 produced from NADPH induces further ROS production by XO, probably in a calcium-dependent manner (8). Though basal expression of XOR is low, factors such as cytokines and oxygen tension could upregulate gene expression. Several in vitro and in vivo studies, demonstrated that factors such as TNF-a, IFN-γ, IL-6 or IL-1 could activate XOR gene transcription (9).
NADPH oxidases catalyze the decrease of molecular oxygen leading to ROS production (10). These oxidases, initially described in neutrophils, are also present in cardiomyocytes, endothelial cells, fibroblasts and smooth muscle cells. The NADPH oxidase consists of two membrane-bound subunits, gp91phox and gp22phox, and three cytosolic proteins p47phox, p22phox and rac1/2. Five NADPH isoforms have been recently identified based on distinct homologues of gp91phox, Nox1-5. Nox2 is the most common NADPH oxidase in the human heart and coexists with Nox1, mainly expressed in VSMC, and Nox4 (11). Activation of Nox is observed in human heart failure, as evidenced by enhanced p47phox staining in the sarcolemmal membrane (12). Furthermore, gp91phox has been shown to be dominantly activated in animal models of cardiac hypertrophy (13). Each Nox may be activated in a different way, eliciting distinct signaling pathways, exhibiting a variety of biochemical properties. Stimuli such as angiotensin-II (Ang-II), endothelin 1 (ET1), TNF-a, noradrenaline as well as mechanical shear stress could induce Rac activation and p47phox phosphorylation, inducing Nox2 expression.
On the other hand, Nox4 activation only requires p22phox, thus seems to be consecutively stimulated by several agonists to produce ROS (11). The Nox4 isotype is expressed in a wide variety of organs, including the heart (14). Nox4 is ubiquitously expressed in various cell types and tissues, including kidneys, the heart, and blood vessels (15). Aside from other members of the Nox family, Nox4 is thought to be constitutively active and does not need cytosolic factors, such as p47phox, p67phox, and the small GTPase Rac, for its activation. Therefore, its expression levels essentially determine the amount of O2– production in cells.
Nitric oxide is a free radical gas, highly diffusible in cell membranes, with vital importance in heart diseases. NO is synthesized by the conversion of L-arginine to L-citrulline, a reaction catalyzed by enzymes called nitric oxide synthases (NOS) with the binding of the critical co-factor tetrahydrobiopterin (BH4). Notably, in pathological conditions, NO reacts with O2– generating peroxynitrite, which in turn could induce the oxidation of BH4. This seems to be the key mechanism for enzyme “uncoupling”, deflecting NOS to greater superoxide production. Furthermore, in the absence of sufficient amounts of substrate, NOS can also produce O2–. Three NOS isoforms are of great importance in myocardium: endothelial NOS (eNOS, or NOS3), inducible NOS (iNOS, or NOS2) and neuronal (nNOS, or NOS1). Recent experimental data demonstrated different intracellular localization of each isoform in cardiomyocytes, as well as altered expression and activity level in the presence of cardiac disease (16).
Endothelial NOS is expressed in coronary arteries and endocardium endothelial cells, in cardiomyocytes and cardiac conducting tissue (16). NO produced by coronary endothelium and cardiomyocytes as well, modulate cardiac contractile function. Furthermore, eNOS activity mainly depends on heart inotropic state and is affected by cardiac contractile function (17). Endothelial NOS uncoupling represents the key mechanism for ROS generation, stimulated in states with increased OS, such as heart failure.
Myocardial nNOS is initially localized in SR (16). Of great interest are specific interactions between nNOS and co-localized XOR in the SR of cardiomyocytes. Interestingly, it has been demonstrated in vitro that inhibition of nNOS expression led to increased O2– generation by XOR. Cardiac mitochondria also contain nNOS. It is possible that mitochondrial nNOS synthesize NO which inhibits cellular respiration, though the exact role is not yet clear (18).
Activated in a calcium-independent manner, iNOS is mainly expressed in cardiac myocytes after stimulation by cytokines in disease states associated with inflammation (19). Circulating cytokines, such as IL-1b, IL-6, TNF-a, and IFN-γ, have been shown to induce iNOS expression, leading to nitrogen reactive species generation, mediating the depressant effects of cytokines in the heart. Besides cardiac myocytes, iNOS is also detected in endocardial and microvascular endothelium, in fibroblasts and vascular smooth muscle cells.
ROS as effectors of intracellular processes
Besides its central role in immune defense against microbial agents, ROS are also involved in cellular signaling pathways (20). ROS mediates the activation of signaling molecules such as nuclear factor-kappa beta (NFkB) and activating protein-1 (AP-1), mitogen-activated protein kinases (MAPK) such as ERK1/2, JNKs and p38 MAPKs (20,21). The modulation of signaling pathways by ROS is defined as “redox signaling”. Exogenous stimuli, such as ET-1, are connected to membrane receptors, mainly G-protein coupled receptors, leading to ROS production and further activation of redox-induced transcription factors, resulting in large-scale effects on cardiac myocytes and subsequently to the human heart (20).
ROS per se have the ability to directly affect the molecular structure and function of important intracellular molecules. They could directly influence the integrity of genomic DNA, leading to crucial mutations, while also they could cause structural modifications to key proteins, leading to enzymatic malfunction or inactivation. Furthermore, ROS affect the intracellular lipids leading to lipid peroxidation, threatening the molecular stability of cellular membrane and cellular organelles.
ROS and redox-regulated pathways
ROS are involved in modulating the activity of specific transcription factors including NFkB and AP-1. NFkB is a well-studied redox-sensitive transcription factor. NFkB inactive form exists in cytosol associated with IkB inhibitory proteins. ROS are involved in modulating the activity of specific transcription factors including NFkB and AP-1. Increased intracellular levels of ROS could lead to NFkB activation via degradation of IkB, which under normal condition inhibits the NFkB transcription. It has been proven that increased intracellular levels of ROS could result in further activation of NFkB, an action mediated by Toll-like receptors (TLRs) (22). Moreover, it has been proposed that the same mechanistic pattern could also count for other redox-sensitive transcription factors such apoptosis signal kinase-1 (ASK-1) (21).
Changes in ROS levels can also affect the function of ion channels, such as calcium (Ca2+) channels, and transporters (23,24). It is known that the function of the cardiac excitation-coupling mechanism is mainly dependent on intracellular Ca2+ levels. ROS by modifying the activity of both the SR release Ca2+ channel (SERCA) and the ryanodine receptor (RyR), directly affect cardiac contraction (23). ROS-mediated alterations in Ca2+ transport systems are involved in various heart disease states, such as ischemia-reperfusion (I/R) injury (23).
Recently, some mechanisms have also been proposed in order to indicate the specific connection between ROS and molecular pathways which regulate important cellular functions. For example, it has been shown that high-mobility group box 1 protein, a nuclear protein bound to chromatin, is induced by increased ROS levels, leading to subsequent NADPH activation, further eliciting downstream pathways leading to cellular apoptosis (25). The same protein, under other circumstances, could have a beneficial role in cellular survival. Recent evidence suggests a significant role for this protein in I/R injury (25). However, it is clear that more studies are needed in order to unravel the complex network connecting ROS to molecular pathways completely.
ROS as signaling molecules
Beyond their role as being the end product of specific molecular changes, ROS could also act as signaling molecule being the bridge connecting the stimuli to the intracellular signaling pathways. Such stimuli could be ATII, FGF-2, TNF-a, ET-1 (21,26). In vivo and in vitro studies have shown that ATII binds to a G protein-coupled receptor on the myocardial cell surface, leading to NOX2 activation and increased ROS production, which subsequently activates factors such as AP-1, triggering the initiation of inflammatory pathways (13,27). Additionally, increased ROS production could mediate TNF-a induced cardiomyocyte apoptosis in vitro (28). Recent data has shown that ET-1 could lead to Ca2+ channels activation via a ROS-mediated pathway (29). In other studies, TNF-a, a common inflammatory cytokine in various cardiovascular diseases, was administered to mice. It was demonstrated that TNF-a induced ventricular remodeling was highly associated with ROS production in ventricles, via a Nox-dependent mechanism (30). Similar mechanisms have been implicated in the b2 adrenoreceptor induced cardiomyopathy and heart failure. Mice overexpressing b2 adrenoreceptor, exhibited higher ROS production, mainly derived from activated NADPH oxidase (31). Subsequently, ROS, acting as a second messenger, induced the activation of p38MAP kinase inflammatory pathway, resulting in cardiac remodeling, cardiomyopathy and heart failure (31).
The role of ROS in cardiac apoptosis
Apoptosis or programmed cell death is a genetically guided physiological mechanism that regulates tissue homeostasis via controlling cell deletion. Clinical and experimental data indicate that cardiomyocyte apoptosis occurs in ischemic and failing hearts and contributes to the cardiac dysfunction and failure. In the last decade, it has been established that ROS could elicit apoptotic pathways in cardiomyocytes. However, the interplay between the molecular factors that participate in this process remains unclear (32).
Stimulation of cardiomyocytes with exogenous ROS causes apoptosis. In adult cardiomyocytes, relatively low levels of H2O2 were found to activate ERK1/2 MAPK pathway, while higher levels also activate JNK, p38 MAPKs and Akt inducing apoptosis (32). Data indicate that ROS also mediate b-adrenergic-induced apoptosis. Stimulation of b-adrenergic receptor, initiated the mitochondrial apoptotic pathway, which involves the release of cytochrome c in the cytosol, as well as the JNK activation, leading to cardiac myocyte apoptosis (33,34). A model of Ang-II-induced apoptosis demonstrated that angiotensin could lead to increased production of ROS, which subsequently led to activation of Ca2+/calmodulin-dependent protein kinase, even at low levels of Ca2+. This, in turn, triggers the activation of p38MAP kinase and apoptosis (35). Data from this study also indicated that NADPH oxidase serves as a source of ROS in this pathway, given that NADPH oxidase inhibition has led to prevention of cardiomyocyte apoptosis (35). Furthermore, the role of NADPH oxidase and especially of its counterpart Rac1 has also been indicated in the hyperglycemia-associated apoptosis (36). High glucose levels lead to increased Rac1 activation and NADPH oxidase-associated ROS production, resulting in cardiomyocyte dysfunction and apoptosis (36). On the other hand, some data describe anti-apoptotic properties of ROS. It has been demonstrated that redox regulated pathways such as JNK pathway, could promote the survival of cardiac myocytes, in a cell culture model of hypoxia-reoxygenation (37). It has been shown that cardiomyocytes transfected with a JNK inhibitory vector, resulted in cells vulnerable to apoptosis (37). Therefore, it has been proposed that the exact role of JNK may be cell-type and upon environmental conditions dependent, a hypothesis which may also be applied to other signaling pathways. However, it is important to mention that different observations, as well as variable experimental results, might be attributed to the different experimental approach used in each study.
Wang et al established for the first time, the role of OS in promoting myocardial fibrosis. The authors demonstrated that KCa3.1 channel is probably a critical target on the OS for its pivotal role in myocardial fibrosis, and the ERK1/2 pathway may be involved in the regulation of OS to KCa3.1 (38). Studies by Somanna et al. and Zhao et al. also confirmed the correlation of Nox4-derived ROS with myocardial fibrosis through AT1 pathway (39) and Akt/mTOR and NFκB pathway (40) respectively. Table 1 summarizes the studies investigating ROS sources, signaling pathways, results and clinical implications.
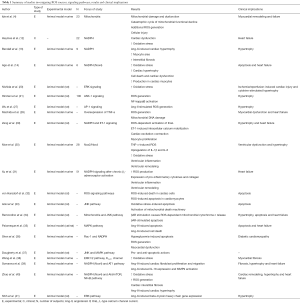
Full table
Conclusions
ROS are molecules that have an oxidizing ability. At physiological concentrations, ROS are vital for normal signal transduction in endothelial cells. Overproduction of ROS or reduced availability of antioxidant enzymes is considered to play a crucial role in the pathogenesis of cardiovascular dysfunction.
ROS are a part of a fundamental mechanism within the cardiomyocyte, which results in cellular OS. OS is a hallmark of various cardiovascular diseases that results in cellular dysfunction and death.
Heart failure and many of the conditions that lead to heart failure are associated with OS. This is considered to be significant in the pathophysiology of the condition, but clinical trials of antioxidant approaches to prevent cardiovascular mortality and morbidity have been unsuccessful. Part of the reason for this may be the failure to appreciate the complexity of the effects of ROS. Excessive OS damages proteins, membranes, and DNA but lower levels of ROS may exert much more subtle and particular regulatory effects (termed redox signaling), even on physiological signaling pathways.
The delineation of specific redox-sensitive pathways and mechanisms that contribute to different components of the failing heart phenotype can facilitate the development of newer targeted therapies as opposed to the failed general antioxidant approaches of the past.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dhalla NS, Das PK, Sharma GP. Subcellular basis of cardiac contractile failure. J Mol Cell Cardiol 1978;10:363-85. [Crossref] [PubMed]
- Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens 2000;18:655-73. [Crossref] [PubMed]
- Sawyer DB, Siwik DA, Xiao L, et al. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Cell Cardiol 2002;34:379-88. [Crossref] [PubMed]
- Ide T, Tsutsui H, Hayashidani S, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res 2001;88:529-35. [Crossref] [PubMed]
- Sawyer DB, Colucci WS. Mitochondrial oxidative stress in heart failure: "oxygen wastage" revisited. Circ Res 2000;86:119-20. [Crossref] [PubMed]
- Weiss JN, Korge P, Honda HM, et al. Role of the mitochondrial permeability transition in myocardial disease. Circ Res 2003;93:292-301. [Crossref] [PubMed]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta 2006;1757:509-17. [Crossref] [PubMed]
- Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 2004;555:589-606. [Crossref] [PubMed]
- Page S, Powell D, Benboubetra M, et al. Xanthine oxidoreductase in human mammary epithelial cells: activation in response to inflammatory cytokines. Biochim Biophys Acta 1998;1381:191-202. [Crossref] [PubMed]
- Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest 2005;115:509-17. [Crossref] [PubMed]
- Sirker A, Zhang M, Murdoch C, et al. Involvement of NADPH oxidases in cardiac remodelling and heart failure. Am J Nephrol 2007;27:649-60. [Crossref] [PubMed]
- Heymes C, Bendall JK, Ratajczak P, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 2003;41:2164-71. [Crossref] [PubMed]
- Bendall JK, Cave AC, Heymes C, et al. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 2002;105:293-6. [Crossref] [PubMed]
- Ago T, Kuroda J, Pain J, et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res 2010;106:1253-64. [Crossref] [PubMed]
- Murdoch CE, Zhang M, Cave AC, et al. NADPH oxidase-dependent redox signalling in cardiac hypertrophy, remodelling and failure. Cardiovasc Res 2006;71:208-15. [Crossref] [PubMed]
- Seddon M, Shah AM, Casadei B. Cardiomyocytes as effectors of nitric oxide signalling. Cardiovasc Res 2007;75:315-26. [Crossref] [PubMed]
- Hattler BG, Oddis C, Zeevi A, et al. Regulation of constitutive nitric oxide synthase activity by the human heart. Am J Cardiol 1995;76:957-9. [Crossref] [PubMed]
- Sears CE, Ashley E, Casadei B. Nitric oxide control of cardiac function: is neuronal nitric oxide synthase a key component? Philos Trans R Soc Lond B Biol Sci 2004;359:1021-44. [Crossref] [PubMed]
- Umar S, van der Laarse A. Nitric oxide and nitric oxide synthase isoforms in the normal, hypertrophic, and failing heart. Mol Cell Biochem 2010;333:191-201. [Crossref] [PubMed]
- Nishida M, Maruyama Y, Tanaka R, et al. G alpha(i) and G alpha(o) are target proteins of reactive oxygen species. Nature 2000;408:492-5. [Crossref] [PubMed]
- Hirotani S, Otsu K, Nishida K, et al. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation 2002;105:509-15. [Crossref] [PubMed]
- Frantz S, Kelly R, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem 2001;276:5197-203. [Crossref] [PubMed]
- Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 2006;71:310-21. [Crossref] [PubMed]
- Lim G, Venetucci L, Eisner DA, et al. Does nitric oxide modulate cardiac ryanodine receptor function? Implications for excitation-contraction coupling. Cardiovasc Res 2008;77:256-64. [Crossref] [PubMed]
- Tang D, Kang R, Zeh HJ, et al. High-mobility group box 1, oxidative stress, and disease. Antioxid Redox Signal 2011;14:1315-35. [Crossref] [PubMed]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation 2003;108:1912-6. [Crossref] [PubMed]
- Wu S, Gao J, Ohlemeyer C, et al. Activation of AP-1 through reactive oxygen species by angiotensin II in rat cardiomyocytes. Free Radic Biol Med 2005;39:1601-10. [Crossref] [PubMed]
- Machida Y, Kubota T. Overexpression of tumor necrosis factor-alpha increases production of hydroxyl radical in murine myocardium. Am J Physiol Heart Circ Physiol 2003;284:H449-55. [Crossref] [PubMed]
- Zeng Q, Zhou Q, Yao F, et al. Endothelin-1 regulates cardiac L-type calcium channels via NAD(P)H oxidase-derived superoxide. J Pharmacol Exp Ther 2008;326:732-8. [Crossref] [PubMed]
- Moe KT, Yin N, Naylynn T, et al. Nox2 and Nox4 mediate tumor necrosis factor-alpha-induced ventricular remodeling in mice. J Cell Mol Med 2011;15:2601-13. [Crossref] [PubMed]
- Xu Q, Dalic A, Fang L, et al. Myocardial oxidative stress contributes to transgenic β2-adrenoreceptor activation-induced cardiomyopathy and heart failure. Br J Pharmacol 2011;162:1012-28. [Crossref] [PubMed]
- von Harsdorf R, Li PF, Dietz R. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. Circulation 1999;99:2934-41. [Crossref] [PubMed]
- Aoki H, Kang PM, Hampe J, et al. Direct activation of mitochondrial apoptosis machinery by c-Jun N-terminal kinase in adult cardiac myocytes. J Biol Chem 2002;277:10244-50. [Crossref] [PubMed]
- Remondino A, Kwon SH, Communal C, et al. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circulation Res 2003;92:136-8. [Crossref] [PubMed]
- Palomeque J, Rueda OV, Sapia L, et al. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circulation Res 2009;105:1204-12. [Crossref] [PubMed]
- Shen E, Li Y, Li Y, et al. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes 2009;58:2386-95. [Crossref] [PubMed]
- Dougherty CJ, Kubasiak LA, Prentice H, et al. Activation of c-Jun N-terminal kinase promotes survival of cardiac myocytes after oxidative stress. Biochem J 2002;362:561-71. [Crossref] [PubMed]
- Wang LP, Fan SJ, Li SM, et al. Oxidative stress promotes myocardial fibrosis by upregulating KCa3.1 channel expression in AGT-REN double transgenic hypertensive mice. Pflugers Arch 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Somanna NK, Valente AJ, Krenz M, et al. The Nox1/4 Dual Inhibitor GKT137831 or Nox4 Knockdown Inhibits Angiotensin-II-Induced Adult Mouse Cardiac Fibroblast Proliferation and Migration. AT1 Physically Associates With Nox4. J Cell Physiol 2016;231:1130-41. [Crossref] [PubMed]
- Zhao QD, Viswanadhapalli S, Williams P, et al. NADPH oxidase 4 induces cardiac fibrosis and hypertrophy through activating Akt/mTOR and NFκB signaling pathways. Circulation 2015;131:643-55. [Crossref] [PubMed]
- Shih NL, Chang T, Loh S, et al. Reactive oxygen species modulate angiotensin II-induced beta-myosin heavy chain gene expression via Ras/Raf/extracellular signal-regulated kinase pathway in neonatal rat cardiomyocytes. Biochem Biophys Res Commun 2001;283:143-8. [Crossref] [PubMed]


