Extracorporeal techniques in acute respiratory distress syndrome
Introduction
Extracorporeal membrane oxygenation (ECMO) has been available to support severe respiratory failure since the 1970s. However, high complication rates, largely due to limitations in technology, lead to poor outcomes early on. In more recent years, advances in technology and management have led to apparently improved survival with reduced complication rates, resulting in increasing use of ECMO for severe acute respiratory distress syndrome (ARDS). While outcomes have improved over time, the benefit of ECMO as compared to conventional, standard of care management for ARDS has yet to be demonstrated in rigorously designed, randomized controlled trials; as such it remains most commonly employed as salvage therapy for the most severe cases of ARDS.
As the field continues to evolve, there is increasing potential for ECMO to enhance the way ARDS is managed, notably through facilitation of lung protective ventilation and minimization of ventilator-associated lung injury. Here we will review the evidence that supports the use of ECMO, the rationale for its use and mechanistic benefits, practical aspects of ECMO initiation and management, and ongoing investigations and future directions.
History of ECMO for ARDS
ECMO is a system that draws blood out of the body through a cannula via a pump, passes the blood through a membrane oxygenator where both oxygen delivery and carbon dioxide removal occur, and reinfuses the well-oxygenated blood back into the body through a cannula, thus providing extracorporeal gas exchange (1).
The first successful use of ECMO as salvage therapy for severe acute respiratory failure was reported in 1972 (2), however, a subsequent randomized controlled trial, published in 1979, failed to demonstrate a survival advantage over conventional mechanical ventilation with low survival rates in both groups (9.5% and 8.3%, respectively) (3). Thereafter, the use of extracorporeal carbon dioxide removal (ECCO2R), which was first observed in the setting of early hemodialysis membranes (4), was recognized as a potential strategy in severe acute respiratory failure, specifically as a means of facilitating carbon dioxide removal and minimization of invasive mechanical ventilation through low frequency positive-pressure ventilation (5-8). Observed survival rates with this strategy were much higher (48.8%) than previously reported in patients with similar clinical characteristics (9), but in a follow-up randomized controlled trial of ECCO2R combined with low frequency positive-pressure ventilation, there was no survival benefit over conventional mechanical ventilation (33% vs. 42%, respectively, P=0.8) (10). Thereafter, a number of observational studies suggested a survival rate ranging from 49–81% (11-19) for selected patients managed with ECMO for severe ARDS. However, conclusions from these early studies on the efficacy of ECMO for ARDS are limited by study methodology and the use of outdated extracorporeal technology as well as mechanical ventilation practices.
In the last 2 decades, there have been a number of changes in clinical practice that have led to improved outcomes in ARDS, most notably the use of a low-volume, low-pressure ventilation strategy, conservative fluid management, neuromuscular blockade, and prone positioning (20-25). Additionally, a number of advances have been made over time in extracorporeal technology, including the use of centrifugal pumps, polymethylpentene membranes, biocompatible circuit components, and improvements in cannula technology (26,27).
The use of ECMO in severe ARDS during the 2009 influenza A (H1N1) pandemic generated more widespread interest in its use, with high overall survival rates (28-30), including a reported survival of 75% in a cohort of patients in Australia and New Zealand (31,32). However, favorable outcomes were also observed for comparable cohorts of patients with severe ARDS due to influenza A (H1N1) at other centers without the use of ECMO (33), calling into question the benefit of ECMO over optimal conventional management. Two cohort studies that utilized matched-pairs analysis of patients with H1N1-asscoiated ARDS who were managed with or without ECMO demonstrated conflicting results; the first reported a mortality benefit (RR 0.45–0.51, P=0.001–0.006) (34), whereas the second did not (OR 1.48, P=0.32) (35).
The only multicenter randomized controlled trial utilizing relatively modern techniques in ECMO for ARDS is the Conventional Ventilation or ECMO for Severe Adult Respiratory Failure (CESAR) trial, in which 180 patients with severe acute respiratory failure were randomized to either receive conventional mechanical ventilation or be referred to a specialized center where they were considered for ECMO after an initial period of optimal conventional management. A significant reduction was seen in the composite outcome of death or severe disability at 6 months in patients who were referred to a specialty center for consideration of ECMO versus conventional management (37% vs. 53%; RR 0.69, 95% CI 0.05–0.97, P=0.03). Of note, only 76% of patients referred to a specialty center were ultimately managed with ECMO, and a large portion of patients in the conventional management arm (30%) never received lung protective ventilation at any time, making it difficult to draw conclusions about the benefit of ECMO itself on outcomes. Despite these and other limitations, referral of patients with severe forms of ARDS to a center that has the capability of performing ECMO, and adheres to standard of care mechanical ventilation, may be beneficial (36,37).
Indications and contraindications for ECMO in ARDS
ECMO may be considered as a salvage therapy for patients with ARDS in those who have severe gas exchange abnormalities in the setting of potentially reversible acute respiratory failure. Proposed thresholds for the initiation of ECMO include severe hypoxemia [e.g., partial pressure of oxygen in arterial blood (PaO2) to the fraction of inspired oxygen (FiO2) ratio less than 80], uncompensated hypercapnia with acidemia (e.g., pH less than 7.15) or excessively high end-inspiratory plateau pressures (e.g., greater than 35–45 cmH2O) despite standard of care low-volume, low-pressure ventilation (1). When available, adjunctive therapies that have a proven or suspected benefit in severe ARDS (e.g., neuromuscular blockade and prone positioning) should be strongly considered prior to the initiation of ECMO.
Patients who have been exposed to high-pressure ventilation (end-inspiratory plateau pressure of greater than 30) or high FiO2 for more than 7 days may be less likely to benefit from ECMO, although clearly this is only a relative contraindication and may simply reflect enrichment of the population for more severe cases. Other relative contraindications include limited vascular access options for cannulation and any condition that does not allow the use of systemic anticoagulation, which is strongly preferred to minimize thrombosis formation in the ECMO circuit (1). Additionally, there are conditions that should exclude patients from receiving ECMO due to the limited overall benefit anticipated from its use. Such patients include those with advanced, untreatable underlying conditions (e.g., irreversible brain injury or metastatic cancer). An absolute contraindication is the use of ECMO in patients with end-stage lung disease and severe acute-on-chronic respiratory failure who are not candidates for lung transplantation when recovery to baseline is not deemed possible (1).
ECMO cannulation and configuration in ARDS
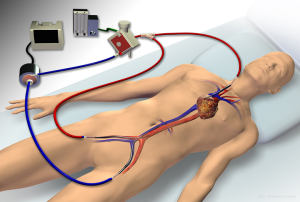
ECMO configurations include venovenous, where deoxygenated blood is drained from a central vein and oxygenated blood is reinfused into a central vein (Figure 1), and venoarterial, where blood is drained from a central vein and reinfused into a central artery. Venovenous ECMO provides respiratory support, whereas venoarterial ECMO provide both respiratory and hemodynamic support. The majority of ARDS cases involve severe respiratory failure alone, for which a venovenous configuration is most appropriate, and is the primary focus of this review. Some cases of ARDS may present with concomitant severe cardiogenic shock. In such circumstances, a hybrid approach, where blood is drained from a vein and reinfusion is split between a central artery and vein (Figure 2), may be necessary to provide both hemodynamic support and adequate upper-body oxygenation (1,38-40). Of note, when ARDS presents with an acute elevation in pulmonary vascular resistance (PVR) with right ventricular dysfunction from severe hypoxemic- or hypercapnic-induced pulmonary vasoconstriction, venovenous ECMO is often effective in improving PVR and unloading the right ventricle such that venoarterial ECMO can be avoided (41).
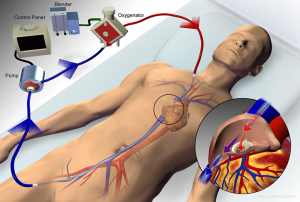
Venovenous ECMO can utilize either a dual-site or single-site cannulation approach. The dual-site approach most commonly drains blood from a femoral vein and reinfuses into an internal jugular or contralateral femoral vein. This approach may be complicated by recirculation of blood, which occurs when reinfused blood is drawn back into the circuit without passing through the systemic circulation, thereby reducing the effectiveness of gas exchange. A single bicaval dual-lumen cannula can also be used (Figure 3), which, when properly positioned, minimizes the likelihood of recirculation and does not require femoral cannulation, allowing for improved mobility of patients when appropriate. However, it does require either transesophageal echocardiography or fluoroscopic guidance to ensure proper positioning, notably directing the reinfusion jet across the tricuspid valve (1,26,42,43).
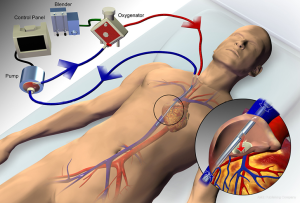
An alternative configuration for gas exchange support is a pumpless, arteriovenous circuit (arterial drainage and venous reinfusion via femoral artery and vein, respectively), which relies upon the patient’s native cardiac output to generate extracorporeal blood flow through the membrane oxygenator. However, the lack of control over extracorporeal blood flow, which tends to be relatively low and thus less effective for oxygenation, and the need for arterial cannulation make this approach less desirable than a pump-based venovenous configuration in most circumstances (44-46).
Management of ECMO in ARDS
Control of oxygenation and carbon dioxide removal
Once a patient is cannulated with venovenous ECMO, a gas supply typically consisting of a mixture of oxygen and air is connected to the membrane oxygenator, with the fraction of delivered oxygen (FDO2) set via a gas blender. This gas, referred to as sweep gas, passes along one side of a semipermeable membrane, while blood flows along the other side, with the membrane allowing for diffusion of oxygen and carbon dioxide down their respective gradients. The sweep gas flow rate is the main determinant of carbon dioxide removal at high blood flow rates, and can be titrated to PaCO2 or pH (Table 1). The level of PaCO2, blood flow rate and properties of the membrane lung also affect carbon dioxide removal.

Full table
The major determinants of blood oxygenation in patients receiving venovenous ECMO include the amount of blood flow through the circuit relative to cardiac output, FDO2 through the circuit, the contribution of native lung gas exchange (which is largely impaired in severe ARDS), and the characteristics of the membrane lung. As such, establishing adequate blood flow based on a patient’s size and predicted cardiac output is an important determinant in choosing the appropriate cannula sizes, with blood flow largely being limited by the size of the drainage cannula. An increase in cardiac output at a given extracorporeal flow rate will decrease systemic oxygenation because of a relative decrease in the contribution of the ECMO circuit to oxygenation. In a small study that evaluated various ECMO parameters with regard to blood oxygenation and decarboxylation in patients with ARDS, it was noted that when blood flow was greater than or equal to 60% of cardiac output, patients were able to maintain an arterial saturation of greater than 90% (47).
Anticoagulation
Systemic anticoagulation is required for all ECMO circuits to minimize the risk of thrombus formation, with unfractionated heparin being the most commonly used anticoagulant. Although there are no universally accepted anticoagulation goals for ECMO, an activated partial thromboplastin time of 40 to 60 seconds has been used by some centers as a target that provides adequate anticoagulation of the circuit while minimizing potential bleeding complications (1,48). Retrospective data suggests that a low level anticoagulation strategy, coupled with conservative transfusion thresholds and reinfusion of circuit blood at the time of decannulation, results in favorable outcomes while minimizing transfusion requirements (49).
Ventilator strategies
While a low-volume, low-pressure strategy is the hallmark of ventilator management in ARDS, (21,50-53) the ideal ventilator settings for patients managed with ECMO are unknown. Secondary analysis of the data from the ARDSNet ARMA trial of low tidal volume ventilation in ARDS suggests patients with even lower end-inspiratory plateau pressures than the targeted 30 cmH2O on day one, had lower mortality rates as compared with those with higher values, regardless of tidal volume assignment, suggesting a lower target may be more protective (53,54). However, the ability to achieve very low plateau airway pressures—below those targeted with a standard of care low-volume, low-pressure ventilation strategy—in patients with ARDS and severely reduced lung compliance, is often limited by unacceptable levels of respiratory acidosis. The concurrent use of ECMO may provide sufficient additional gas exchange support to allow for further reductions in ventilator volumes and pressures while managing the hypercapnia and acidemia that accompanies the reduction in minute ventilation. Whether this strategy is superior to standard of care low-volume, low-pressure ventilation is unknown.
The CESAR trial managed patients with pressure-controlled ventilation with a target peak inspiratory pressure of 20–25 cmH2O, a rate of 10 breaths per minute, PEEP of 10–15 cmH2O and FiO2 of 0.3 (36). This strategy has often been adopted in the practice of ECMO. However, the ventilator strategy itself was not tested. Multiple approaches may be acceptable, including the use of volume-cycled ventilation to target a particular plateau airway pressure, although the optimal plateau airway pressure has yet to be determined. Prospective ARDS studies suggest that there may not be a safe upper limit of tidal volume or plateau airway pressure (53). In addition, recent reports highlight the role of respiratory rate as a contributor to ventilator-associated lung injury (55,56) and that lower respiratory rates (e.g., lower than 10 breaths per minute) should be considered. Analysis of pooled data of patients managed with mechanical ventilation alone for ARDS and those managed with venovenous ECMO have also suggested that driving pressure (plateau airway pressure minus positive end-expiratory pressure) is independently associated with increased mortality; while this relationship has not been validated in a prospective or randomized fashion, perhaps targeting a lower driving pressure could also be beneficial (57,58).
In light of the significant mortality benefit seen with the use of prone positioning in patients with ARDS (25), prone positioning should be strongly considered prior to the initiation of ECMO, when possible (59). Prone positioning might also be considered in selected patients managed with ECMO. However, little is known about the effects of combining these strategies. One small case series reviewed the outcomes in patients with ARDS who were managed with the combination of ECMO and prone positioning. The authors noted improved oxygenation and general safety of the procedure with no complications attributable to prone positioning (60).
Extubation during extracorporeal support
Given that the fundamental goals for ventilator management in ARDS are geared towards minimizing ventilator-associated lung injury, removing the ventilator entirely may theoretically be the preferred strategy. Additionally, it could optimize other intensive care-based management strategies, including minimization of sedation, reductions in nosocomial infections (particularly ventilator-associated pneumonia) and maximization of mobilization and enteral nutrition. However, there is potential concern over exacerbating mechanical stress with spontaneous breathing in ARDS (61-65). Although ECCO2R has been shown to have the ability to control ventilatory drive in select patients with severe, chronic respiratory failure (e.g., COPD), data suggests that it may not be able to sufficiently control the spontaneous and potentially injurious respiratory efforts of patients with severe ARDS (66,67).
Mobilization during extracorporeal support
Physical and occupational therapy has been shown to not only be feasible, but also have a number of favorable outcomes in patients with acute respiratory failure, notably improving functionality, reducing delirium, and increasing ventilator-free days (68-70). The mobilization of patients with respiratory failure requiring ECMO has been increasing overall, but this data is largely limited to patients who are awaiting lung transplantation (71-74). While patients requiring ECMO for ARDS may often be too critically ill to participate in active rehabilitation, it may be possible in appropriate patients who are at centers that have a multidisciplinary approach to physical therapy (75-77). The benefit of mobilizing ARDS patients on ECMO has not been well defined and must be weighed against the potential risks of physical therapy in this population.
Complications
Potential risks or complications of ECMO include hemorrhage, thrombosis, hemolysis, and infection, among others, and must be weighed against the potential benefits when selecting appropriate patients for ECMO support (78-82). A lower anticoagulation goal has been adopted by many centers in an attempt to minimize the risks of hemorrhage while still maintaining circuit patency (1). Advances in extracorporeal technology and techniques and increasing experience have reduced the rates of these complications over time, however, the risks of ECMO remain considerable (78).
Economics of ECMO for ARDS
There are limited data evaluating the cost-benefit profile of ECMO in ARDS. The CESAR trial incorporated an economic evaluation within their study, which noted more than a two-fold increase in cost per patient who received ECMO as compared to standard management, with a gain of 0.03 quality-adjusted life-years (QALYs) at 6 months. However, this was limited to one health care system in the context of a randomized controlled trial (36,83). Further economic assessments will be important as the use of this technology continues to widen.
Ethical considerations
ECMO has the ability to support gas exchange for patients with severe respiratory failure. However, ethical dilemmas have arisen, most notably when patients are unable to be weaned from extracorporeal support and are deemed not to be candidates for lung transplantation. There are currently no available devices that would offer a “destination therapy” or more portable extracorporeal devices that would allow such a patient with persistent respiratory failure the option of residing outside the ICU. As such, families and patients may find themselves in a situation with no clear endpoint to their clinical course (84). Careful selection of patients who have a higher likelihood of a favorable outcome from ARDS may help avoid some of these dilemmas (85-87). Potential future technological advances in device therapy may one day help remedy such situations by providing a durable destination device.
Ongoing areas of investigation
ECCO2R for less severe ARDS
While the use of ECCO2R was first described in the 1970’s as an alternative means of providing ventilation, pursuit of novel management strategies incorporating ECCO2R have become more popular in recent years in response to the increasingly recognized importance of lung-protective ventilatory strategies and advances in technology that have improved the risk-benefit profile of extracorporeal support. Venovenous ECMO has largely been reserved as salvage therapy for cases of the most severe forms of ARDS (1,88), the incidence of which is low compared to less severe forms of ARDS (89). As such, there is increasing interest in the possible utilization of ECCO2R in less severe cases of ARDS in an attempt to facilitate or extend low-volume, low-pressure ventilation, which is often otherwise limited by hypercapnia with acidemia. The removal of carbon dioxide via an extracorporeal circuit, notably with lower flow and smaller cannulae (which is more feasible in patients with less severe hypoxemia), could permit the optimization of lung protective strategies, including lower tidal volumes, plateau airway pressures, and respiratory rates, by maintaining pH within an acceptable range. This concept of ECCO2R-assisted very-low tidal volume ventilation in patients with ARDS was studied in a prospective trial that used ECCO2R to reduce tidal volumes from 6 mL/kg of predicted body weight to approximately 4 mL/kg with a goal of reducing plateau airway pressure from 28–30 cmH2O to 25–27 cmH2O. In doing so, inflammatory markers (including interleukin 6, interleukin 8, interleukin 1b, and interleukin 1 receptor antagonist) were significantly reduced from baseline, suggesting potential mitigation of ventilator-associated lung injury (90). A subsequent randomized controlled trial compared the use of ECCO2R-assisted very-low tidal volume ventilation (tidal volume of 3 mL/kg predicted body weight) to a standard lung protective ventilation strategy in patients with moderate to severe ARDS. Although there was no difference in the primary outcome of ventilator free days at 28 and 60 days between the two groups (33 vs. 29, P=0.469), there was a suggestion of benefit among those with more severe hypoxemia (91). Based on the limited literature that exists, extending lung protective ventilation to very-low tidal volume ventilation is achievable with the assistance of ECCO2R. The overall clinical benefits of such a strategy are still uncertain and need to be further elucidated in randomized-controlled trials.
Conclusions
Despite a lack of rigorous, high-quality evidence, modern-day ECMO is increasingly becoming accepted as a reasonable salvage therapy for patients with severe ARDS (92), and has the ability to maximize lung-protective ventilation in these patients, albeit with uncertain benefit. The application of ECCO2R may, in the future, also play an important role in the mitigation of ventilator-associated lung injury in less severe forms of ARDS. Randomized controlled trials are needed to assess the potential impact of extracorporeal technology on the management of ARDS.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Brodie is currently on the medical advisory boards of ALung Technologies and Kadence. All compensation for these activities is paid to Columbia University. Dr. Parekh and Dr. Abrams have no conflicts of interest to declare.
References
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Hill JD, O'Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med 1972;286:629-34. [Crossref] [PubMed]
- Zapol WM, Snider MT, Hill JD, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 1979;242:2193-6. [Crossref] [PubMed]
- Sherlock JE, Yoon Y, Ledwith JW, et al. Respiratory gas exchange during hemodialysis. Proc Clin Dial Transplant Forum 1972;2:171-4. [PubMed]
- Gattinoni L, Kolobow T, Agostoni A, et al. Clinical application of low frequency positive pressure ventilation with extracorporeal CO2 removal (LFPPV-ECCO2R) in treatment of adult respiratory distress syndrome (ARDS). Int J Artif Organs 1979;2:282-3. [PubMed]
- Gattinoni L, Kolobow T, Damia G, et al. Extracorporeal carbon dioxide removal (ECCO2R): a new form of respiratory assistance. Int J Artif Organs 1979;2:183-5. [PubMed]
- Gattinoni L, Agostoni A, Pesenti A, et al. Treatment of acute respiratory failure with low-frequency positive-pressure ventilation and extracorporeal removal of CO2. Lancet 1980;2:292-4. [Crossref] [PubMed]
- Gattinoni L, Pesenti A, Caspani ML, et al. The role of total static lung compliance in the management of severe ARDS unresponsive to conventional treatment. Intensive Care Med 1984;10:121-6. [Crossref] [PubMed]
- Gattinoni L, Pesenti A, Mascheroni D, et al. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA 1986;256:881-6. [Crossref] [PubMed]
- Morris AH, Wallace CJ, Menlove RL, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 1994;149:295-305. [Crossref] [PubMed]
- Wagner PK, Knoch M, Sangmeister C, et al. Extracorporeal gas exchange in adult respiratory distress syndrome: associated morbidity and its surgical treatment. Br J Surg 1990;77:1395-8. [Crossref] [PubMed]
- Brunet F, Belghith M, Mira JP, et al. Extracorporeal carbon dioxide removal and low-frequency positive-pressure ventilation. Improvement in arterial oxygenation with reduction of risk of pulmonary barotrauma in patients with adult respiratory distress syndrome. Chest 1993;104:889-98. [Crossref] [PubMed]
- Kolla S, Awad SS, Rich PB, et al. Extracorporeal life support for 100 adult patients with severe respiratory failure. Ann Surg 1997;226:544-64; discussion 65-6. [Crossref] [PubMed]
- Lewandowski K, Rossaint R, Pappert D, et al. High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med 1997;23:819-35. [Crossref] [PubMed]
- Manert W, Haller M, Briegel J, et al. Venovenous extracorporeal membrane oxygenation (ECMO) with a heparin-lock bypass system. An effective addition in the treatment of acute respiratory failure (ARDS). Anaesthesist 1996;45:437-48. [PubMed]
- Peek GJ, Moore HM, Moore N, et al. Extracorporeal membrane oxygenation for adult respiratory failure. Chest 1997;112:759-64. [Crossref] [PubMed]
- Bartlett RH, Roloff DW, Custer JR, et al. Extracorporeal life support: the University of Michigan experience. JAMA 2000;283:904-8. [Crossref] [PubMed]
- Lindén V, Palmér K, Reinhard J, et al. High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med 2000;26:1630-7. [Crossref]
- Schmid C, Philipp A, Hilker M, et al. Venovenous extracorporeal membrane oxygenation for acute lung failure in adults. J Heart Lung Transplant 2012;31:9-15. [Crossref] [PubMed]
- Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 1998;338:347-54. [Crossref] [PubMed]
- Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Villar J, Kacmarek RM, Perez-Mendez L, et al. A high positive end-expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Crit Care Med 2006;34:1311-8. [Crossref] [PubMed]
- Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 2006;354:2564-75. [Crossref] [PubMed]
- Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010;363:1107-16. [Crossref] [PubMed]
- Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013;368:2159-68. [Crossref] [PubMed]
- Wang D, Zhou X, Liu X, et al. Wang-Zwische double lumen cannula-toward a percutaneous and ambulatory paracorporeal artificial lung. ASAIO J 2008;54:606-11. [Crossref] [PubMed]
- Bottrell S, Bennett M, Augustin S, et al. A comparison study of haemolysis production in three contemporary centrifugal pumps. Perfusion 2014;29:411-6. [Crossref] [PubMed]
- Patroniti N, Zangrillo A, Pappalardo F, et al. The Italian ECMO network experience during the 2009 influenza A(H1N1) pandemic: preparation for severe respiratory emergency outbreaks. Intensive Care Med 2011;37:1447-57. [Crossref] [PubMed]
- Roch A, Lepaul-Ercole R, Grisoli D, et al. Extracorporeal membrane oxygenation for severe influenza A (H1N1) acute respiratory distress syndrome: a prospective observational comparative study. Intensive Care Med 2010;36:1899-905. [Crossref] [PubMed]
- Holzgraefe B, Broome M, Kalzen H, et al. Extracorporeal membrane oxygenation for pandemic H1N1 2009 respiratory failure. Minerva Anestesiol 2010;76:1043-51. [PubMed]
- Davies A, Jones D, Bailey M, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA 2009;302:1888-95. [Crossref] [PubMed]
- Davies A, Jones D, Gattas D. Extracorporeal Membrane Oxygenation for ARDS Due to 2009 Influenza A(H1N1)—Reply. JAMA 2010;303:941-2. [Crossref]
- Miller RR 3rd, Markewitz BA, Rolfs RT, et al. Clinical findings and demographic factors associated with ICU admission in Utah due to novel 2009 influenza A(H1N1) infection. Chest 2010;137:752-8. [Crossref] [PubMed]
- Noah MA, Peek GJ, Finney SJ, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA 2011;306:1659-68. [Crossref] [PubMed]
- Pham T, Combes A, Roze H, et al. Extracorporeal Membrane Oxygenation for Pandemic Influenza A(H1N1)-induced Acute Respiratory Distress Syndrome: A Cohort Study and Propensity-matched Analysis. Am J Respir Crit Care Med 2013;187:276-85. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]
- Zwischenberger JB, Lynch JE. Will CESAR answer the adult ECMO debate? Lancet 2009;374:1307-8. [Crossref] [PubMed]
- Biscotti M, Lee A, Basner RC, et al. Hybrid configurations via percutaneous access for extracorporeal membrane oxygenation: a single-center experience. Asaio j 2014;60:635-42. [Crossref] [PubMed]
- Bréchot N, Luyt CE, Schmidt M, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock*. Crit Care Med 2013;41:1616-26. [Crossref] [PubMed]
- Abrams D, Brodie D. Emerging indications for extracorporeal membrane oxygenation in adults with respiratory failure. Ann Am Thorac Soc 2013;10:371-7. [Crossref] [PubMed]
- Reis Miranda D, van Thiel R, Brodie D, et al. Right ventricular unloading after initiation of venovenous extracorporeal membrane oxygenation. Am J Respir Crit Care Med 2015;191:346-8. [Crossref] [PubMed]
- Javidfar J, Brodie D, Wang D, et al. Use of bicaval dual-lumen catheter for adult venovenous extracorporeal membrane oxygenation. Ann Thorac Surg 2011;91:1763-8; discussion 9.
- Javidfar J, Wang D, Zwischenberger JB, et al. Insertion of bicaval dual lumen extracorporeal membrane oxygenation catheter with image guidance. ASAIO J 2011;57:203-5. [Crossref] [PubMed]
- Bein T, Weber F, Philipp A, et al. A new pumpless extracorporeal interventional lung assist in critical hypoxemia/hypercapnia. Crit Care Med 2006;34:1372-7. [Crossref] [PubMed]
- Fischer S, Simon AR, Welte T, et al. Bridge to lung transplantation with the novel pumpless interventional lung assist device NovaLung. J Thorac Cardiovasc Surg 2006;131:719-23. [Crossref] [PubMed]
- Aziz F, Brehm CE, El-Banyosy A, et al. Arterial Complications in Patients Undergoing Extracorporeal Membrane Oxygenation via Femoral Cannulation. Ann Vasc Surg 2014;28:178-83. [Crossref] [PubMed]
- Schmidt M, Tachon G, Devilliers C, et al. Blood oxygenation and decarboxylation determinants during venovenous ECMO for respiratory failure in adults. Intensive Care Med 2013;39:838-46. [Crossref] [PubMed]
- Sy E, Sklar MC, Lequier L, et al. Anticoagulation practices and the prevalence of major bleeding, thromboembolic events, and mortality in venoarterial extracorporeal membrane oxygenation: A systematic review and meta-analysis. J Crit Care 2017;39:87-96. [Crossref] [PubMed]
- Agerstrand CL, Burkart KM, Abrams DC, et al. Blood conservation in extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Thorac Surg 2015;99:590-5. [Crossref] [PubMed]
- Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med 2005;33:1-6; discussion 230-2. [Crossref] [PubMed]
- Ranieri VM, Suter PM, Tortorella C, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282:54-61. [Crossref] [PubMed]
- Putensen C, Theuerkauf N, Zinserling J, et al. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med 2009;151:566-76. [Crossref] [PubMed]
- Needham DM, Colantuoni E, Mendez-Tellez PA, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ 2012;344:e2124. [Crossref] [PubMed]
- Hager DN, Krishnan JA, Hayden DL, et al. Tidal volume reduction in patients with acute lung injury when plateau pressures are not high. Am J Respir Crit Care Med 2005;172:1241-5. [Crossref] [PubMed]
- Gattinoni L, Tonetti T, Cressoni M, et al. Ventilator-related causes of lung injury: the mechanical power. Intensive Care Med 2016;42:1567-75. [Crossref] [PubMed]
- Grasso S, Stripoli T, Mazzone P, et al. Low respiratory rate plus minimally invasive extracorporeal Co2 removal decreases systemic and pulmonary inflammatory mediators in experimental Acute Respiratory Distress Syndrome. Crit Care Med 2014;42:e451-60. [Crossref] [PubMed]
- Amato MBP, Meade MO, Slutsky AS, et al. Driving Pressure and Survival in the Acute Respiratory Distress Syndrome. N Engl J Med 2015;372:747-55. [Crossref] [PubMed]
- Serpa Neto A, Schmidt M, Azevedo LC, et al. Associations between ventilator settings during extracorporeal membrane oxygenation for refractory hypoxemia and outcome in patients with acute respiratory distress syndrome: a pooled individual patient data analysis. Intensive Care Med 2016;42:1672-84. [Crossref] [PubMed]
- Brodie D, Guerin C. Rescue therapy for refractory ARDS should be offered early: no. Intensive Care Med 2015;41:926-9. [Crossref] [PubMed]
- Guervilly C, Hraiech S, Gariboldi V, et al. Prone positioning during veno-venous extracorporeal membrane oxygenation for severe acute respiratory distress syndrome in adults. Minerva Anestesiol 2014;80:307-13. [PubMed]
- Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 2013;188:1420-7. [Crossref] [PubMed]
- Güldner A, Pelosi P, Gama de Abreu M. Spontaneous breathing in mild and moderate versus severe acute respiratory distress syndrome. Curr Opin Crit Care 2014;20:69-76. [Crossref] [PubMed]
- Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med 2017;195:438-42. [Crossref] [PubMed]
- Nosotti M, Rosso L, Tosi D, et al. Extracorporeal membrane oxygenation with spontaneous breathing as a bridge to lung transplantation. Interact Cardiovasc Thorac Surg 2013;16:55-9. [Crossref] [PubMed]
- Mauri T, Bellani G, Grasselli G, et al. Patient-ventilator interaction in ARDS patients with extremely low compliance undergoing ECMO: a novel approach based on diaphragm electrical activity. Intensive Care Med 2013;39:282-91. [Crossref] [PubMed]
- Mauri T, Langer T, Zanella A, et al. Extremely high transpulmonary pressure in a spontaneously breathing patient with early severe ARDS on ECMO. Intensive Care Med 2016;42:2101-3. [Crossref] [PubMed]
- Crotti S, Bottino N, Ruggeri GM, et al. Spontaneous Breathing during Extracorporeal Membrane Oxygenation in Acute Respiratory Failure. Anesthesiology 2017;126:678-87. [Crossref] [PubMed]
- Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874-82. [Crossref] [PubMed]
- Needham DM, Korupolu R. Rehabilitation quality improvement in an intensive care unit setting: implementation of a quality improvement model. Top Stroke Rehabil 2010;17:271-81. [Crossref] [PubMed]
- Bailey P, Thomsen GE, Spuhler VJ, et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med 2007;35:139-45. [Crossref] [PubMed]
- Turner DA, Cheifetz IM, Rehder KJ, et al. Active rehabilitation and physical therapy during extracorporeal membrane oxygenation while awaiting lung transplantation: a practical approach. Crit Care Med 2011;39:2593-8. [Crossref] [PubMed]
- Rehder KJ, Turner DA, Hartwig MG, et al. Active rehabilitation during extracorporeal membrane oxygenation as a bridge to lung transplantation. Respir Care 2013;58:1291-8. [Crossref] [PubMed]
- Javidfar J, Brodie D, Iribarne A, et al. Extracorporeal membrane oxygenation as a bridge to lung transplantation and recovery. J Thorac Cardiovasc Surg 2012;144:716-21. [Crossref] [PubMed]
- Abrams DC, Brenner K, Burkart KM, et al. Pilot study of extracorporeal carbon dioxide removal to facilitate extubation and ambulation in exacerbations of chronic obstructive pulmonary disease. Ann Am Thorac Soc 2013;10:307-14. [Crossref] [PubMed]
- Abrams D, Javidfar J, Farrand E, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care 2014;18:R38. [Crossref] [PubMed]
- Munshi L, Kobayashi T, DeBacker J, et al. Intensive care physiotherapy during extracorporeal membrane oxygenation for acute respiratory distress syndrome. Ann Am Thorac Soc 2017;14:246-53. [PubMed]
- Ko Y, Cho YH, Park YH, et al. Feasibility and Safety of Early Physical Therapy and Active Mobilization for Patients on Extracorporeal Membrane Oxygenation. Asaio j 2015;61:564-8. [Crossref] [PubMed]
- Paden ML, Conrad SA, Rycus PT, et al. Extracorporeal Life Support Organization Registry Report 2012. Asaio j 2013;59:202-10. [Crossref] [PubMed]
- Schmidt M, Brechot N, Hariri S, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis 2012;55:1633-41. [Crossref] [PubMed]
- Heilmann C, Geisen U, Beyersdorf F, et al. Acquired von Willebrand syndrome in patients with extracorporeal life support (ECLS). Intensive Care Med 2012;38:62-8. [Crossref] [PubMed]
- Cheng R, Hachamovitch R, Kittleson M, et al. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: a meta-analysis of 1,866 adult patients. Ann Thorac Surg 2014;97:610-6. [Crossref] [PubMed]
- Johnson SM, Itoga N, Garnett GM, et al. Increased risk of cardiovascular perforation during ECMO with a bicaval, wire-reinforced cannula. J Pediatr Surg 2014;49:46-49; discussion 49-50. [Crossref]
- Peek GJ, Elbourne D, Mugford M, et al. Randomised controlled trial and parallel economic evaluation of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR). Health Technol Assess 2010;14:1-46. [Crossref] [PubMed]
- Abrams DC, Prager K, Blinderman CD, et al. Ethical dilemmas encountered with the use of extracorporeal membrane oxygenation in adults. Chest 2014;145:876-82. [Crossref] [PubMed]
- Schmidt M, Zogheib E, Roze H, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med 2013;39:1704-13. [Crossref] [PubMed]
- Schmidt M, Bailey M, Sheldrake J, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med 2014;189:1374-82. [Crossref] [PubMed]
- Enger T, Philipp A, Videm V, et al. Prediction of mortality in adult patients with severe acute lung failure receiving veno-venous extracorporeal membrane oxygenation: a prospective observational study. Crit Care 2014;18:R67. [Crossref] [PubMed]
- Extracorporeal Life Support Organization. ECLS registry report, international summary. Available online: www.elso.org
- Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med 2005;353:1685-93. [Crossref] [PubMed]
- Terragni PP, Del Sorbo L, Mascia L, et al. Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 2009;111:826-35. [Crossref] [PubMed]
- Bein T, Weber-Carstens S, Goldmann A, et al. Lower tidal volume strategy (approximately 3 ml/kg) combined with extracorporeal CO(2) removal versus “conventional” protective ventilation (6 ml/kg) in severe ARDS: The prospective randomized Xtravent-study. Intensive Care Med 2013;39:847-56. [Crossref] [PubMed]
- Morris AH. Exciting new ECMO technology awaits compelling scientific evidence for widespread use in adults with respiratory failure. Intensive Care Med 2012;38:186-8. [Crossref] [PubMed]


