Prognostic value of admission red blood cell distribution width in acute pancreatitis: a systematic review
Introduction
Acute pancreatitis (AP) is one of the most common gastrointestinal cause of hospital admissions in US with approximately 275,000 hospitalizations in the year 2009 and annual incidence of up to 13–45/100,000 persons (1). Clinical manifestation and effects of AP, range from a mild, self-limited disease to severe and sometimes fatal disease. However, reported mortality from AP is about 1% but this risk increases with age, co-morbidities and development of complications, and varies from 7–42% in severe disease (1). Early identification of these patients who are at high risk of mortality in emergency room can help us with rational use of more aggressive treatment leading to decreased mortality rate (1). Therefore, there is a need for simple, easily obtainable and inexpensive markers to determine the prognosis of AP. In previous studies, several AP scoring systems and laboratory tests have been proposed and developed to estimate the prognosis of AP, such as Ranson’s score, Balthazar score, BISAP score and SIRS score, C-reactive protein (CRP), serum blood urea nitrogen (BUN), D-dimer and pro-calcitonin levels (2). However, there are multiple disadvantages associated with score systems such as hassle of calculation and need for ordering specific tests (2).
Red blood cell distribution width (RDW) is an easily obtained, inexpensive, routinely reported parameter as a part of the complete blood count test. It is commonly performed in the assessment of almost all the patients at the time of admission (3). Conventionally, RDW has been used as a tool to explore the etiologies of anemia (3). During the past decade, however, accumulated studies have shown that RDW is associated with the risk, disease activity and prognosis of various diseases, such as malignancies (4), heart failure (5), autoimmune diseases (6) and hepatocellular carcinoma (7) etc.
To date, multiple studies have investigated the usefulness of RDW in determining the prognosis of AP at the time of admission, but the results have not been consistent. Our aim is to perform a systematic literature review to summarize the published evidences from available studies on use of RDW at the time of admission to predict prognosis and mortality in AP.
Methods
Literature retrieval
We searched Medline (using PubMed as search engine), Cochrane, Google scholar, and Web of Science in March 02, 2017 to identify studies investigating the association between RDW and AP. The search algorithms used in PubMed were: [“red blood cell distribution width” or “red cell distribution width” or “RDW” or Erythrocyte Indices (mesh)]and pancreatitis. Similar strategies were used in Web of Science. Additionally, the references of each article were also searched for relevant citations.
Study selection and data extraction
After all the potential studies were retrieved, we performed a title and abstract screening to exclude irrelevant studies. For the remaining studies, a full text review was performed to justify the eligibility of the study. We only included studies investigating the prognostic value of RDW in AP patients, with outcomes of all-cause mortality, hospital mortality, pancreatitis specific mortality, intensive care unit (ICU) supervision, hospital length of stay, severity of disease, or presence of organ failure. Non-English publications were excluded.
The following data were extracted from eligible studies: first author, sources of subjects, publication year, sample size, type of data collection (prospective or retrospective), outcomes studied, mortality rate, area under receiver operating characteristic (ROC) curve (AUC) and its 95% confidence interval (95% CI), whether multivariable analysis was performed, and adjustment for confounding factors.
Results
Study selection process
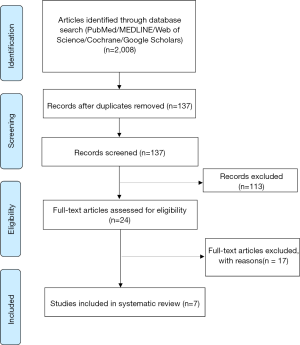
Figure 1 is a flowchart of study selection. After screening and assessment, seven of them met the eligibility criteria and were included in our systematic review (9-15).
Summary of eligible studies
The characteristics of eligible studies are summarized in Table 1. The sample size of eligible studies ranged from 102 to 359 (9-15). Three studies were performed in Turkey (9,11,12), three studies (13-15) were performed in China, and one study (9) was based on clinical database named Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC II) from USA (16). All of the studies were retrospective design except one study did not report the type of data collection (13). The outcomes studied by the eligible studies included hospital mortality (9-11), AP mortality (13), and mortality within 48 hours (12) or within three months (14,15). The mortality rate ranges from 4.3% to 13.3%. All studies reported that the non-survivors have significantly higher admission RDW compared to survivors. Five studies (10,11,13-15) evaluated the prognostic value of RDW using ROC curve analysis, and three reported AUC values higher than 0.80 (11,13,14). While in the MIMIC II database study, the AUC was 0.66 (10).

Full table
By comparing the clinical characteristics of those who died vs. those who survived during hospitalization, the studies found that age, renal function, calcium and white blood cell count (WBC) were potential predictors for mortality (Table 1). However, only five studies analyzed the association between RDW and mortality using multivariable analysis (9-12,15), and four studies (9-11,15) reported that RDW was independently associated with mortality. The common confounding factors adjusted for in multivariable analysis including age (9,10,12,15), WBC (9,11), albumin (9,11), BUN (9,11), creatinine (10), calcium (9,11), platelet count (9,11). None of the studies investigated the association between RDW and ICU care, hospital length of stay or presence of organ failure. One study investigated the association between RDW and severity of AP and found that RDW is increased in severe acute pancreatitis (SAP) (15).
Discussion
This systematic review identified seven retrospective studies which investigated the prognostic value of RDW in AP. Employing different methodology and various RDW cut-off values, all of the included studies demonstrated that admission RDW significantly predicted clinical outcomes and mortality in AP. However, these studies, only adjusted for a limited range of potential confounding factors.
Using ROC curve analyses, three studies reported that the AUC of RDW for predicting mortality is more than 0.80. It is noteworthy that, also by using ROC curve analysis, many studies have investigated the predictive value of some well-recognized score systems in AP, including acute physiology and chronic health evaluation (APACHE) score (17), bedside index of severity in AP (BISAP) (18), Ranson score (19), Glasgow score (20), and the AUCs of these score systems are approximately 0.80 (20-26) which is comparable to that of RDW. These results indicate that RDW is a strong prognostic factor for AP. Additionally, five studies analyzed the association between RDW and mortality of AP using multivariable analysis, and four of them found that RDW is independently associated with mortality in AP. Taken together; these studies indicated that RDW has a utility in estimating the prognosis of AP.
The underlying pathophysiologic mechanism of association between RDW and prognosis of AP remains unclear. We postulate that the prognostic value of RDW in AP is mediated by inflammation response. This hypothesis is also supported by some previous studies. First, previous studies have suggested that RDW is an inflammatory marker and is positively correlated with inflammatory markers in unselected outpatients (27) and apparently healthy individuals (28). Second, it is well-accepted that inflammation impairs the bone marrow function, iron metabolism and erythrocyte homeostasis (29). Increased inflammatory cytokines such as tumor necrosis factor α, interleukin-1 and interleukin-6 due to sepsis in AP, have been shown to suppress maturation of erythrocytes leading to entry of larger reticulocytes in the peripheral blood (30) causing elevation in RDW. Third, inflammatory markers such as CRP (31), pro-calcitonin (32,33) and tumor necrosis factor (TNF) (34,35) have been shown/used as a prognostic marker in AP. Fourth, Ucar Karabulut et al. retrospectively analyzed patient with AP and found that RDW value was significantly higher during the bout of AP when compared to the samples obtained after complete recovery (36). Taken together, these studies support that inflammation, at least partially, mediates the association between RDW and prognosis of AP.
During the past decades, several scoring systems have been proposed for early identification of increased morbidity, outcomes and mortality in AP such as acute physiologic assessment and chronic health evaluation II (APACHE II) score (17), systemic inflammatory response syndrome (SIRS) score (37), bedside index of severity in acute pancreatitis (BISAP) (18), Glasgow score (20) and sequential organ failure assessment (SOFA) (38). However, when compared with these scoring systems, RDW has its strengths. First, RDW is routinely ordered as part of a complete blood count (CBC), and is easily obtained without any additional costs (39). Second, it is readily available and easy to use in comparison to score systems as no calculations are needed.
Although available studies have indicated that RDW is a useful index for estimating the prognosis of AP, some limitations of these studies are worth mentioning. First, confounding factors should be considered when performing observational studies. Only five studies (9-12,15) adjusted for the effects of confounding factors using multivariable analyses, and four studies (9-11,15) reported that RDW is independently associated with mortality after potential confounding factors have been adjusted. However, most of the studies that adjusted for confounders might have failed to adjust for potential confounders including gender (40,41), unrecognized deficiency of iron, vitamins B12, and folate (42). Besides, RDW is also affected directly by alcohol intake which is the second most common cause of AP (42).
Second, all the available studies are observational and retrospective in design, and therefore, the reliability of the results is greatly affected by the representativeness of the subjects. Further studies with prospective design, with appropriate samples are needed to rigorously evaluate the prognostic value of RDW in AP.
Third, all of the studies included in the review have investigated the predictive value of RDW for hospital mortality, but none have investigated the prognostic value of RDW to predict the severity of AP or persistent organ failure. Additionally, the association between RDW and ICU care (admission or transfer), or length of hospital stay, were not studied. Further studies examining the possible prognostic value of RDW for predicting various outcomes related to AP are warranted.
In conclusion, the available evidences support that RDW is a useful index for predicting mortality in patients with AP. Future prospective studies with larger samples, robust analyses, and examining various outcomes related to AP are needed to rigorously evaluate the prognostic value of RDW in AP.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013;144:1252-61. [Crossref] [PubMed]
- Gomatos IP, Xiaodong X, Ghaneh P, et al. Prognostic markers in acute pcreatitis. Expert Rev Mol Diagn 2014;14:333-46. [Crossref] [PubMed]
- Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med 1991;9 Suppl 1:71-4. [Crossref] [PubMed]
- Montagnana M, Danese E. Red cell distribution width and cancer. Ann Transl Med 2016;4:399. [Crossref] [PubMed]
- Huang YL, Hu ZD, Liu SJ, et al. Prognostic value of red blood cell distribution width for patients with heart failure: A systematic review and meta-analysis of cohort studies. PLoS One 2014;9:e104861. [Crossref] [PubMed]
- Hu ZD. Red blood cell distribution width: a promising index for estimating activity of autoimmune disease. J Lab Precis Med 2016;1:4. [Crossref]
- Goyal H, Hu ZD. Prognostic value of red blood cell distribution width in hepatocellular carcinoma. Ann Transl Med 2017;5:271. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Cetinkaya E, Senol K, Saylam B, et al. Red cell distribution width to platelet ratio: new and promising prognostic marker in acute pancreatitis. World J Gastroenterol 2014;20:14450-4. [Crossref] [PubMed]
- Hu ZD, Wei TT, Tang QQ, et al. Prognostic value of red blood cell distribution width in acute pancreatitis patients admitted to intensive care units: an analysis of a publicly accessible clinical database MIMIC II. Clin Chem Lab Med 2016;54:e195-7. [Crossref] [PubMed]
- Şenol K, Saylam B, Kocaay F, et al. Red cell distribution width as a predictor of mortality in acute pancreatitis. Am J Emerg Med 2013;31:687-9. [Crossref] [PubMed]
- Gülen B, Sonmez E, Yaylaci S, et al. Effect of harmless acute pancreatitis score, red cell distribution width and neutrophil/lymphocyte ratio on the mortality of patients with nontraumatic acute pancreatitis at the emergency department. World J Emerg Med 2015;6:29-33. [Crossref] [PubMed]
- Yao J, Lv G. Association between red cell distribution width and acute pancreatitis: a cross-sectional study. BMJ Open 2014;4:e004721. [Crossref] [PubMed]
- Wang D, Yang J, Zhang J, et al. Red cell distribution width predicts deaths in patients with acute pancreatitis. J Res Med Sci 2015;20:424-8. [Crossref] [PubMed]
- Li Y, Zhao Y, Feng L, et al. Comparison of the prognostic values of inflammation markers in patients with acute pancreatitis: a retrospective cohort study. BMJ Open 2017;7:e013206. [Crossref] [PubMed]
- Saeed M, Villarroel M, Reisner AT, et al. Multiparameter Intelligent Monitoring in Intensive Care II: a public-access intensive care unit database. Crit Care Med 2011;39:952-60. [Crossref] [PubMed]
- Ke L, Tong ZH, Li WQ, et al. Predictors of critical acute pancreatitis: a prospective cohort study. Medicine (Baltimore) 2014;93:e108. [Crossref] [PubMed]
- Wu BU, Johannes RS, Sun X, et al. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut 2008;57:1698-703. [Crossref] [PubMed]
- Ranson JH, Rifkind KM, Roses DF, et al. Prognostic signs and the role of operative management in acute pancreatitis. Surg Gynecol Obstet 1974;139:69-81. [PubMed]
- Tan YH, Rafi S, Tyebally Fang M, et al. Validation of the modified Ranson versus Glasgow score for pancreatitis in a Singaporean population. ANZ J Surg 2015. [Epub ahead of print]. [Crossref] [PubMed]
- Koziel D, Gluszek S, Matykiewicz J, et al. Comparative analysis of selected scales to assess prognosis in acute pancreatitis. Can J Gastroenterol Hepatol 2015;29:299-303. [Crossref] [PubMed]
- Garcea G, Gouda M, Hebbes C, et al. Predictors of severity and survival in acute pancreatitis: validation of the efficacy of early warning scores. Pancreas 2008;37:e54-61. [Crossref] [PubMed]
- Singh VK, Wu BU, Bollen TL, et al. A prospective evaluation of the bedside index for severity in acute pancreatitis score in assessing mortality and intermediate markers of severity in acute pancreatitis. Am J Gastroenterol 2009;104:966-71. [Crossref] [PubMed]
- Park JY, Jeon TJ, Ha TH, et al. Bedside index for severity in acute pancreatitis: comparison with other scoring systems in predicting severity and organ failure. Hepatobiliary Pancreat Dis Int 2013;12:645-50. [Crossref] [PubMed]
- Valverde-López F, Matas-Cobos AM, Alegria-Motte C, et al. BISAP, RANSON, lactate and others biomarkers in prediction of severe acute pancreatitis in a European cohort. J Gastroenterol Hepatol 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Cho YS, Kim HK, Jang EC, et al. Usefulness of the Bedside Index for severity in acute pancreatitis in the early prediction of severity and mortality in acute pancreatitis. Pancreas 2013;42:483-7. [Crossref] [PubMed]
- Lippi G, Targher G, Montagnana M, et al. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med 2009;133:628-32. [PubMed]
- Vayá A, Sarnago A, Fuster O, et al. Influence of inflammatory and lipidic parameters on red blood cell distribution width in a healthy population. Clin Hemorheol Microcirc 2015;59:379-85. [Crossref] [PubMed]
- Fraenkel PG. Anemia of Inflammation: A Review. Med Clin North Am 2017;101:285-96. [Crossref] [PubMed]
- Morceau F, Dicato M, Diederich M. Pro-inflammatory cytokine-mediated anemia: regarding molecular mechanisms of erythropoiesis. Mediators Inflamm 2009;2009:405016. [Crossref] [PubMed]
- Mäkelä JT, Eila H, Kiviniemi H, et al. Computed tomography severity index and C-reactive protein values predicting mortality in emergency and intensive care units for patients with severe acute pancreatitis. Am J Surg 2007;194:30-4. [Crossref] [PubMed]
- Pindak D, Parrak V, Pechan J, et al. The clinical value of the procalcitonin in prediction of severity and outcome in acute pancreatitis. Hepatogastroenterology 2003;50 Suppl 2:ccviii-ccix. [PubMed]
- Rau BM, Kemppainen EA, Gumbs AA, et al. Early assessment of pancreatic infections and overall prognosis in severe acute pancreatitis by procalcitonin (PCT): a prospective international multicenter study. Ann Surg 2007;245:745-54. [Crossref] [PubMed]
- Chen CC, Wang SS, Lee FY, et al. Proinflammatory cytokines in early assessment of the prognosis of acute pancreatitis. Am J Gastroenterol 1999;94:213-8. [Crossref] [PubMed]
- Surbatovic M, Radakovic S. Tumor necrosis factor-alpha levels early in severe acute pancreatitis: is there predictive value regarding severity and outcome? J Clin Gastroenterol 2013;47:637-43. [Crossref] [PubMed]
- Uçar Karabulut K, Narci H, Ucar Y, et al. Association between red blood cell distribution width and acute pancreatitis. Med Sci Monit 2014;20:2448-52. [Crossref] [PubMed]
- Gregoric P, Sijacki A, Stankovic S, et al. SIRS score on admission and initial concentration of IL-6 as severe acute pancreatitis outcome predictors. Hepatogastroenterology 2010;57:349-53. [PubMed]
- Juneja D, Gopal PB, Ravula M. Scoring systems in acute pancreatitis: which one to use in intensive care units? J Crit Care 2010;25:358.e9-15. [Crossref] [PubMed]
- Hu ZD, Wei TT, Zhong RQ. Red blood cell distribution: an index without additional cost in estimating the prognosis of acute pancreatitis. Clin Chem Lab Med 2016;54:e389-90. [Crossref] [PubMed]
- Lippi G, Salvagno GL, Guidi GC. Red blood cell distribution width is significantly associated with aging and gender. Clin Chem Lab Med 2014;52:e197-9. [PubMed]
- Hoffmann JJ, Nabbe KC, van den Broek NM. Effect of age and gender on reference intervals of red blood cell distribution width (RDW) and mean red cell volume (MCV). Clin Chem Lab Med 2015;53:2015-9. [Crossref] [PubMed]
- Goyal H, Gupta S, Singla U. Level of red cell distribution width is affected by various factors. Clin Chem Lab Med 2016;54:e387. [Crossref] [PubMed]


