Lung cancer screening with low dose CT and radiation harm—from prediction models to cancer incidence data
Lung cancer is the first neoplastic killer worldwide (1). In 2011 the results of the National Lung Screening Trial (NLST) in US indicated a 20% lung cancer mortality reduction in subjects at high risk undergoing annual screening low dose computed tomography (LDCT) as compared to chest X-ray (2). A 11–30% reduction of lung cancer mortality was recently reported in three small randomized clinical trials (RCT) in Italy, Europe (3,4), propelling adoption of LDCT in subjects at risk of lung cancer.
One general concern about lung cancer screening with LDCT is the potential harm associated with exposition to ionizing radiations employed in the LDCT screening test and in some investigations commonly used for work-up of suspicious lesions discovered at LDCT. These include follow-up with LDCT, 2-(18F)flu-2-deoxy-D-glucose positron emission tomography (FDG-PET) alone or combined with CT for attenuation correction (FDG-PET/CT), contrast-enhanced or unenhanced full-dose CT and CT-guided fine needle aspiration (FNA) or core biopsy, representing up to 22.6% of the overall radiation dose in a LDCT screening program (5). In particular, overall ionizing radiations exposure associated with the LDCT screening procedure may imply an increased incidence of solid cancers and leukemia.
The study by Rampinelli et al. recently published in the BMJ (6) significantly adds to previous risk-benefit analyses of LDCT lung cancer screening (5,7-11). The study is based on the detailed calculation of the radiation dose in a population of volunteers recruited in the COSMOS observational (one arm) LDCT study in Italy, who underwent annual LDCT screening for 10 years that represents one of the longest screening period. As in previous studies, the cumulative radiation dose in terms of effective dose (12,13) was then used to compute the lifetime attributable risk of cancer incidence based on the Biological Effects of Ionizing Radiation VII (BEIR VII) report (14).
In particular, 5,203 participants (66% men and 34% women) aged 50 and older, who were current or former smokers (≥6% men and 34% women) underwent 42,228 LDCT and 635 FDG-PET/CT scans over 10 years. Individual and cumulative radiation exposure from LDCT and FDG-PET/CT scans were calculated by dosimetry software. The median cumulative effective dose at the 10th year of screening was 9.3 mSv for men and 13.0 mSv for women. According to participants’ age and sex, the lifetime attributable risk of lung cancer after 10 years of CT screening ranged from 5.5 to 1.4 per 10,000 people screened, and the lifetime attributable risk of major cancers from 8.1 to 2.6 per 10,000 people screened: in women aged 50–54, the lifetime attributable risk of lung cancer was about four-fold and the risk of major cancers three-fold higher than for men of 65 years and older. The calculated numbers of lung cancer and major cancer cases induced by 10 years of screening in the COSMOS study cohort were 1.5 and 2.4, respectively, which corresponded to an additional risk of induced major cancers of 0.05% (2.4/5,203). In 10 years of screening 259 lung cancers were diagnosed in the COSMOS study. Hence one radiation induced major cancer would be expected for every 108 (259/2.4) lung cancers detected through screening. The Authors concluded that “radiation exposure and cancer risk associated with lung cancer LDCT screening are not negligible, but are acceptable due to the substantial mortality reduction obtained with screening”.
Risk-benefit analyses
Risk-benefit analyses that focus on the balance between number of radiation-induced cancers and number of potentially treatable lung cancers discovered by LDCT screening are based on a series of assumptions. Moreover several factors that influence the analysis are continuously modifying the scenarios.
Assumptions
While the benefit of LDCT screening is being defined (2-4), the risk of radiation induced cancer is not established and controversial (8,15).
In NLST the LDCT screening test involved an approximate dose of 2 mSv, whereas full-chest CT scanning that was the major diagnostic study used to follow up nodules, involved a dose of about 8 mSv (11). However, the average individual effective dose associated with LDCT test and other procedures used for diagnostic-work up in two Italian studies (in which LDCT was used for follow-up of suspicious nodules) was lower and in the range between 0.9 and 1.7 mSv per year (5,6).
Notably, the models for estimates of radiation-induced cancers are based on the dose measurements and observational data about cancer incidence in two cohorts. The first is represented by atomic bomb survivors entailing single exposure to high doses (12-14). The second is constituted by nuclear energy industry workers receiving continuous low-moderate doses for a cumulative amount in the range of 100–600 mGy that corresponds to 100–600 mSv since absorbed dose was largely due to external irradiation (16,17). Hence the estimates refer to radiation doses which are about 100 to 600 times higher than those associated with both single LDCT screening test and the above average individual dose in participants to LDCT screening studies. The estimates of radiation-induced cancers proposed by different Institutions for a given effective dose are not identical (5).
Moreover, the cancer risk from low-level radiation such as that used for diagnostic radiology procedures has been extrapolated by the above observations obtained at moderate and high doses using a linear no-threshold relation between the radiation dose and the risk of cancer (15). However, based on observational, experimental, and radiobiological data, the possibility that the linear no-threshold relation underestimates or overestimates the cancer risk from a low radiation dose, the presence of a dose threshold below which the risk is 0, and even a possible protective effect of low-radiation dose against cancer (so-called hormetic response) should also be considered. When, cautiously, a linear no-threshold dose-risk relation is adopted, other assumptions are necessary for some variables that can modify the dose–risk theoretical relationship including age at exposure, the temporal profile of the exposure and the latent period between exposure and increased cancer incidence.
In particular, the risk of low-dose radiation-induced cancer incidence generally decreases with advancing age. It is noteworthy, however, that the risk of radiation-induced lung cancer is different with a peak at 50–60 years of age (8). An acute exposure is associated with higher risk of cancer than that of protracted exposures of the same total dose (8). Notably, data in airline crew did not show an excess of cancer incidence (except skin cancers) and of mortality despite a continuous exposure to low dose radiations in the range of 2–5 mSv per year (18,19). Finally, the latent period between radiation exposure and cancer death increases with decreasing exposure, and it is possible that for low doses the latent period exceeds the normal life span (15).
Factors influencing risk-benefit analyses
Several factors can profoundly modify the risk-benefit scenarios, including the individual risk profiles, the methodology of screening procedure, and the CT technology and technical options (Table 1).

Full table
Individual risks profiles
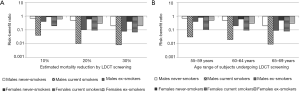
The benefit and potential harm of LDCT lung cancer screening are fundamentally linked to the individual profiles concerning, on the one hand, the probability to develop lung cancer and, on the other hand, the susceptibility to cancerogenic effects of ionizing radiations. Accordingly, a greater risk of lung cancer is associated with smoking history and age and additional factors as asbestos exposure, while the role of gender in modifying the risk is probable but currently undefined. The risk of radiation induced cancer is inversely proportional to the age at exposure and is greater for women (14). The complex interplay among age, smoking history and gender on the risk-benefit estimates for three levels (10%, 20% and 30%) of lung cancer mortality reduction associated with LDCT screening is exemplified in Figure 1. Not surprisingly, the harm of cancer development may outweigh the benefit of lung cancer detection in case of young (below 55 years of age) non-smokers, especially if women (10). This fully justifies efforts in the a priori identification of the subjects who are likely to benefit from LDCT through risk prediction modelling and of subjects to whom screening should not be offered.
Screening procedure
So far lung cancer LDCT screening studies were generally performed by inviting participating subjects to annual LDCT for a variable period (number of annual screening rounds). The optimal duration of active screening is not established and may theoretically extend to life-time (8,11). In such a case it was estimated from the NLST data that a 55-year-old lung screening participant may experience a cumulative radiation exposure of up to 280 mSv over a 20-year period and 420 mSv over 30 years (11). These exposures are in the range of those of nuclear workers and atomic bomb survivors.
However, alternative designs have been explored including extending the interval between LDCT rounds to 2 years (20,21) and even obtaining one single (one shot) LDCT round (22), both contributing to substantially decrease the radiation exposure.
As mentioned above the management of suspicious lesions in LDCT screening is variable and usually entails choice among short (1 or 3 months) follow-up LDCT, FDG-PET, FDG-PET/CT, contrast-enhanced or unenhanced full dose CT, CT-guided diagnostic FNA or core biopsy, which are associated to additional radiation exposure.
CT technology
The dose associated with LDCT screening test intrinsically reflects the CT scanner technology. In ITALUNG single detector spiral CT scanner delivered an almost double dose (1.1 mSv for whole lung 3 mm thick sections) than multiple (4) rows of detectors spiral CT scanner (0.59 mSv for whole lung 1 mm sections) (9). With multiple rows of detector scanner technology available today, doses below 1 mSv are delivered for LDCT screening test with whole lung 1 mm or thinner sections.
Ultra-low-dose CT (ULCT) enabling reduction of the radiation dose to 1/10 of that of LDCT has been developed by applying new iterative reconstruction algorithms. ULCT was recently applied to lung cancer screening (23).
CT technique
Variables influencing radiation dose include X-ray collimation, mAs and kV of the X-ray tube. Decrease of the collimation implies decrease of the signal to noise ratio and increase of the radiation dose to cover the entire lung volume, whereas increase of the mAs and kV is associated with increase of the signal to noise ratio and of the dose (9).
Perspective
The incidence of breast cancer rates associated with different types and amount of radiation exposure and temporal profile of the dose exposure was measured in a 15–30 years range of follow-up from eight different large cohorts, none of which within breast or other screening settings (24). The results supported the linearity of the radiation dose response for breast cancer and suggested that the risks for acute and fractioned high-dose-rate exposures were similar, whereas effects from low-dose-rate protracted exposures were much lower.
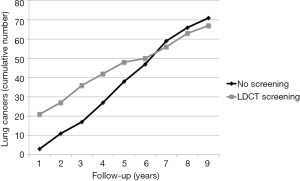
Lung cancer screening with LDCT has been initiated since almost 20 years in Japan (25). Hence, theoretically, based on the latency period estimates of radiation induced cancer (14), individuals screened in the 1990s may have entered the period of actual development and manifestation of radiation induced cancers. Differently from observational studies, RCTs of LDCT lung cancer screening are intrinsically capable to provide real data about the incidence of an excess of radiation induced cancers, especially if the control population undergoes usual care rather than chest X-rays. Remarkably this excess would translate into an increased incidence of lung cancers in the screened population and ultimately a higher risk-benefit ratio. In this perspective, hopefully pooled analysis of the incidence and mortality data in the screened vs. control cohorts in RCTs of lung cancer screening with LDCT is worthy. In the meantime it is of potential interest that follow-up data in the ITALUNG RCT demonstrated that after 6 years since randomization, the 1,406 actively screened subjects began to show a lower cumulative incidence of lung cancer as compared to the 1,593 controls undergoing usual care (Figure 2) (4). Obviously, both longer follow-up and greater sample sizes are necessary to confirm this observation, as well investigation of the incidence of other cancers beside those of the lung and in particular breast cancer. However, this preliminary observation seems reassuring and may open the way to solve the issue of risk of radiation induced neoplasms associated with LDCT screening of lung cancer with collection of real incidence rather than projection data based on risk/benefit modelling.
In conclusion the study by Rampinelli et al. (6) represents a valuable advance to the traditional way to address the issue of potential radiation induced cancers in LDCT screening that is to perform risk-benefit model analyses. In fact it was based on the detailed computation of individual radiation exposure using dedicated software and on a long screening period. The data confirm the successful efforts of the industry and radiologists to contain radiation dose in LDCT screening of lung cancer according to the “as low as reasonably achievable” (ALARA) principle of dose containment. Cancer incidence and mortality follow-up data of RCT investigating efficacy of LDCT screening of lung cancer are expected in the next years to provide real vs. projection data about the putative risk of radiation induced cancer associated with the LDCT screening procedure.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Infante M, Sestini S, Galeone C, et al. Lung cancer screening with low-dose spiral computed tomography: evidence from a pooled analysis of two Italian randomized trials. Eur J Cancer Prev 2017;26:324-9. [Crossref] [PubMed]
- Paci E, Puliti D, Lopes Pegna A, et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Mascalchi M, Mazzoni LN, Falchini M, et al. Dose exposure in the ITALUNG trial of lung cancer screening with low-dose CT. Br J Radiol 2012;85:1134-9. [Crossref] [PubMed]
- Rampinelli C, De Marco P, Origgi D, et al. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356:j347. [Crossref] [PubMed]
- Nishizawa K, Iwai K, Matsumoto T, et al. Estimation of the exposure and a risk–benefit analysis for a CT system designed for a lung cancer mass screening unit. Radiat Prot Dosimetry 1996;67:101-8. [Crossref]
- Brenner DJ. Radiation risks potentially associated with low-dose CT screening of adult smokers for lung cancer. Radiology 2004;231:440-5. [Crossref] [PubMed]
- Mascalchi M, Belli G, Zappa M, et al. Risk-benefit analysis of X-ray exposure associated with lung cancer screening in the Italung-CT trial. AJR Am J Roentgenol 2006;187:421-9. [Crossref] [PubMed]
- Berrington de González A, Kim KP, Berg CD. Low-dose lung computed tomography screening before age 55: estimates of the mortality reduction required to outweigh the radiation-induced cancer risk. J Med Screen 2008;15:153-8. [Crossref] [PubMed]
- McCunney RJ, Li J. Radiation risks in lung cancer screening programs: a comparison with nuclear industry workers and atomic bomb survivors. Chest 2014;145:618-24. [Crossref] [PubMed]
- The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann ICRP 2007;37:1-332. [PubMed]
- ICRP. Radiation dose to patients from radiopharmaceuticals. Addendum 3 to ICRP Publication 53. ICRP Publication 106. Approved by the Commission in October 2007. Ann ICRP 2008;38:1-197. [PubMed]
- National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII-phase 2. Washington, DC: National Academies Press, 2006.
- Cohen BL. Cancer risk from low-level radiation. AJR Am J Roentgenol 2002;179:1137-43. [Crossref] [PubMed]
- Jacob P, Rühm W, Walsh L, et al. Is cancer risk of radiation workers larger than expected? Occup Environ Med 2009;66:789-96. [Crossref] [PubMed]
- Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ 2015;351:h5359. [Crossref] [PubMed]
- Pukkala E, Aspholm R, Auvinen A, et al. Incidence of cancer among Nordic airline pilots over five decades: occupational cohort study. BMJ 2002;325:567. [Crossref] [PubMed]
- Hammer GP, Auvinen A, De Stavola BL, et al. Mortality from cancer and other causes in commercial airline crews: a joint analysis of cohorts from 10 countries. Occup Environ Med 2014;71:313-22. [Crossref] [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [Crossref] [PubMed]
- Pastorino U, Rossi M, Rosato V, et al. Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. Eur J Cancer Prev 2012;21:308-15. [Crossref] [PubMed]
- Baldwin DR, Duffy SW, Wald NJ, et al. UK Lung Screen (UKLS) nodule management protocol: modelling of a single screen randomised controlled trial of low-dose CT screening for lung cancer. Thorax 2011;66:308-13. [Crossref] [PubMed]
- Fujita M, Higaki T, Awaya Y, et al. Lung cancer screening with ultra-low dose CT using full iterative reconstruction. Jpn J Radiol 2017;35:179-89. [Crossref] [PubMed]
- Preston DL, Mattsson A, Holmberg E, et al. Radiation effects on breast cancer risk: a pooled analysis of eight cohorts. Radiat Res 2002;158:220-35. [Crossref] [PubMed]
- Kaneko M, Eguchi K, Ohmatsu H, et al. Peripheral lung cancer: screening and detection with low-dose spiral CT versus radiography. Radiology 1996;201:798-802. [Crossref] [PubMed]


