Balanced versus isotonic saline resuscitation—a systematic review and meta-analysis of randomized controlled trials in operation rooms and intensive care units
Introduction
Stabilization of hemodynamics is a key intervention in patients with shock, and usually requires intravenous infusion of fluids (1). Worldwide isotonic saline is the most commonly used solution for fluid resuscitation (2). Dilutional or hyperchloremic metabolic acidosis is a well-known side-effect of infusion of large amounts of isotonic saline, which could be prevented with the use of balanced crystalloid solutions (3,4).
Balanced crystalloid solutions more closely resemble the electrolyte composition of plasma (5). The potential clinical benefit from resuscitation with balanced solutions comes from the lower chloride content, as development of dilutional acidosis could increase the inflammatory response (6,7), and decrease splanchnic perfusion (8), myocardial contractility (9) and the cardiovascular catecholamine response (10). It is highly uncertain, however, whether prevention of dilutional acidosis has an effect on clinically relevant outcomes in patients who need fluid resuscitation for shock. Moreover, balanced solutions contain different buffers, such as lactate, acetate, or gluconate, which all could have side-effects when infused in large amounts (11).
We performed a meta-analysis of published randomized controlled trials (RCTs) comparing fluid resuscitation with balanced solutions versus isotonic saline in patients either in the operation room or in the intensive care unit (ICU). We hypothesized that fluid resuscitation with balanced solutions is associated with higher in-hospital survival, less development of acute kidney injury (AKI) and less need for renal replacement therapy (RRT). We also determined whether fluid resuscitation with balanced solutions was associated with changes in serum chloride, and arterial pH.
Methods
Search strategy
Studies were identified through an electronic search in PubMed (1966 to February 2016) and CENTRAL (the Cochrane Library to February 2016) using a sensitive search strategy incorporating keywords as well as Medical Subject Headings. Details of the search strategy are reported in Supplementary files and Table S1. All articles identified by the search were scanned for relevancy by title and abstract. For potentially relevant articles the full text was obtained for review; for these articles, all references were inspected and potentially relevant titles were hand searched. The search had no limitations.

Full table
Selection of studies
We restricted the search to (I) RCTs; (II) comparing fluid resuscitation with a balanced solution versus isotonic saline; (III) in adult patients (age >18 years) in (IV) operation room or ICUs. We excluded observational studies, studies conducted exclusively in emergency departments (EDs), studies in pregnant, and trials in which fluid resuscitation with a balanced solution was compared with resuscitation with a colloid solution. Colloid infusion before start of a trial was not a reason for exclusion. Two independent researchers (A.S.N. and R.B.K.) performed the search and results were entered into a database. Wherever these researchers disagreed, this was settled by discussion or by including a third researcher (I.M.L.). The Cochrane Risk of Bias Tool was used to assess the quality of the studies.
Endpoints
The primary endpoint was in-hospital mortality at longest follow-up, which was defined as death during hospital stay. Secondary outcomes were development of AKI, need for RRT, ICU—and hospital length of hospital, and the incidence of metabolic acidosis and changes in plasma chloride levels are described.
Statistical analysis
We expressed pooled dichotomous data and pooled continuous effect measures as odds ratio (OR) or standardized mean difference (SMD) with a 95% confidence intervals (95% CI). A random-effects model was used for all analyses. Trials in ICUs were separated from trials in operation room, as the prognosis is very different in these patient categories, and different outcomes with respect to the primary endpoint, in-hospital mortality, were suspected.
The homogeneity assumption was measured by the I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance. I2 was calculated from basic results obtained from a typical meta-analysis as I2=100% × (Q − df)/Q, where Q is the Cochran heterogeneity statistic. A value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity. All analyses were conducted stratified according to location of patients (ICU vs. operation room).
Parametric variables were presented as the mean ± SD and non-parametric variables were presented as the median (interquartile range). All analyses were conducted with Review Manager v.5.1.1, SPSS v.20 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corporation) or R v.2.12.0 (R Foundation for Statistical Computing, Vienna, Austria). For all analyses two-sided P<0.05 were considered significant.
Results
Search results
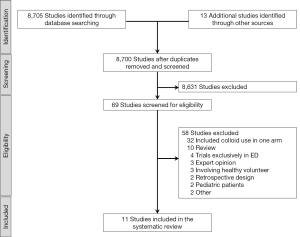
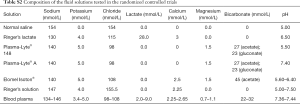
The initial search yielded 8,718 articles (940 from MEDLINE, and 1,258 from CENTRAL). After removing duplicates, the abstracts of 8,700 articles were evaluated. We excluded 8,631 articles because they did not meet the inclusion criteria. Subsequently, the full text of the remaining 69 articles was obtained. Fifty-eight articles were excluded after full text review due to following reasons: included colloid or other solutions in one of the trial arms (n=32); reviews (n=10); trials conducted exclusively in EDs (n=4); not an RCT (n=5); involving healthy volunteers (n=3), RCT in pediatric patients (n=2), RCT in a non–human setting (n=1) and article retracted (n=1). Finally, 11 RCTs (2,703 participants) were included in the final analysis (Figure 1) (12-22). The composition of the balanced solutions tested in each RCT is shown in Table S2.
Full table
Description of studies
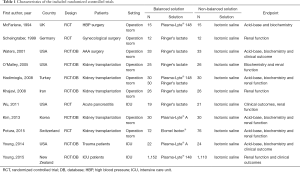
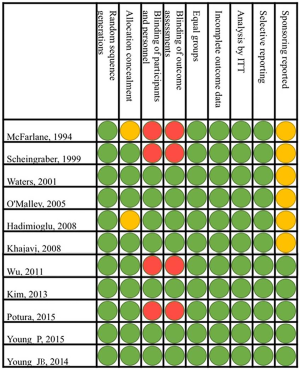
The RCTs were published between 1994 and 2015 and included operation room patients in eight and ICU patients in three RCTs (Table 1). The methodological quality of the RCTs is shown in Figure S1. In all trials the random sequence generation was described and only in two trials the description of the allocation concealment was unclear (Figure S1). Regarding blinding of participants, personnel and assessments, four trials were considered at high risk of bias (Figure S1).
Full table
Full table
All RCTs used isotonic saline for fluid resuscitation in the control arm. Infused amounts of fluids and duration of fluid infusions are shown in Table S3. In operation room patients, the total amount of fluids used for resuscitation varied from 1.5 to 7.0 liters; in ICU patients the total amount of fluids used for resuscitation varied from 2.6 to 10.3 liters.
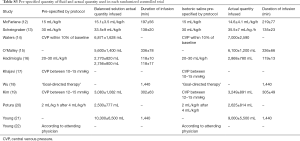
Full table
Primary outcome
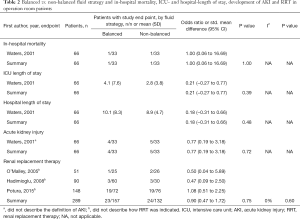
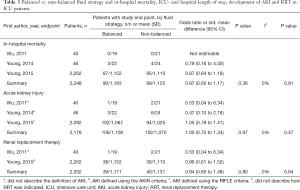
In operation room patients, one out of 33 patients (3.0%) receiving balanced solutions and one out of 33 patients (3.0%) receiving isotonic saline died during hospital stay (OR, 1.00; 95% CI, 0.06–16.69; P=1.00; one study included in the analysis) (Table 2). In ICU patients, 90 out of 1,193 patients (7.5%) receiving balanced solutions and 99 out of 1,155 patients (8.6%) receiving isotonic saline died during hospital stay (OR, 0.87; 95% CI, 0.65–1.17; P=0.36; three studies included in the analysis) (Table 3). There was no sign of heterogeneity (I2=0%).
Full table
Full table
Secondary outcomes
In operation room patients, there were no differences in subsequent ICU or hospital length of stay for patients receiving balanced solution compared to isotonic saline (one study included in the analysis, Table 2). Available information was not sufficient for a meta-analysis of ICU and hospital length of stay in ICU patients.
In operation room patients, four out of 33 patients (12.1%) receiving balanced solutions and five out of 33 patients (15.1%) receiving isotonic saline developed AKI during hospital stay (OR, 0.77; 95% CI, 0.19–3.18; P=0.72; one study included in the analysis) (Table 2). In ICU patients, 106 out of 1,108 patients (9.6%) receiving balanced solutions and 102 out of 1,070 patients (9.5%) receiving isotonic saline developed AKI during hospital stay (OR, 1.00; 95% CI, 0.75–1.34; P=0.97; three studies included in the analysis) (Table 3). There were no differences in the need of RRT, neither in operation room patients (three studies included in the analysis, Table 2) nor in ICU patients (one study included in the analysis, Table 3). There was no sign of heterogeneity (I2=0%).
Metabolic status
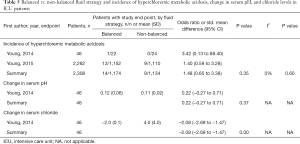
The incidence of metabolic acidosis, as reported in six RCTs, was not different between the two randomized groups, neither in operation room nor in ICU patients (Tables 4,5). Changes in arterial pH after fluid resuscitation were also similar between the two types of fluids, both in operation room and ICU patients (Tables 4,5). In both patient groups there was a larger increase in the chloride levels after fluid resuscitation with isotonic saline compared to balanced solutions (Tables 4,5).
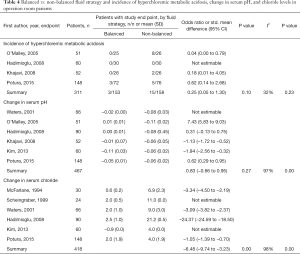
Full table
Full table
Discussion
The present meta-analysis of RCTs comparing fluid resuscitation with balanced solutions with isotonic saline for shock treatment did not find a difference in in-hospital mortality, occurrence of AKI or need for RRT. Compared to resuscitation with isotonic saline, resuscitation with balanced solutions was associated with a smaller increase in the chloride levels. These effects were not different between operation room and ICU patients. One silent finding was that the number of high quality RCTs published on this topic is surprisingly low, seen the frequency with which these fluids are administered in operation room and ICU patients.
The strength of this meta-analysis is the approach of a systematic review and meta-analysis method by only including RCTs. Retrospective or observational studies (23) were excluded, as such preventing the risk for bias. Also studies that compared balanced solutions along with colloids and gelatins (24,25). The RCTs used in the meta-analysis were all of high quality, and included operation room as well as ICU patients allowing comparisons in the two patient groups those were most often subjected to fluid resuscitation.
Intravenous fluids are by far the most frequently administered drugs in the operation room and ICUs. Compositions of intravenous fluids have been and remain to be a matter of debate in both patient populations. Based on the non-physiological composition of ‘normal saline’, the interest to find a fluid that will provide the optimal composition has moved from synthetic colloid solutions to ‘more physiologic’ balanced solutions. The hypothetical benefit of resuscitation with balanced solutions is prevention of dilutional acidosis. By definition, balanced fluids are those that contain an electrolyte constitution that more closely resemble plasma concentrations; they impose a lower chloride-load but contain buffering agents. In experimental models of sepsis, occurrence of hyperchloremia is associated with an increased pro-inflammatory state (6,7), suggesting an immunomodulatory effect. In healthy volunteers, hyperchloremia is associated with a decrease in mean renal artery flow velocity and renal cortical tissue perfusion (26), and an increased time to urination and decreased urine production (26,27). Observational studies in ICU patients suggest an increased mortality in association with hyperchloremia (28), coagulopathy (29), decreased organ perfusion (30) and gastro-intestinal symptoms (8), as well as increased need for transfusion of blood products (24,31,32). Thus, balanced solutions could have a strong potential to affect outcome of patients with shock, who are frequently suffering from a pro-inflammatory and pro-coagulant state.
The results of the present systematic review and meta-analysis add to our understanding of the role of balanced solutions in fluid resuscitation by suggesting that major outcome measures do not differ between following fluid resuscitation with balanced and isotonic saline solutions, both in operation room and ICU patients. More RCTs are needed to show whether resuscitation with balanced or isotonic solutions affects important clinical endpoints before a recommendation for one type of fluid can be made. Actually, from the results of this systematic review and meta-analysis one could conclude that currently evidence insufficiently supports the use of balanced over isotonic saline for fluid resuscitation to improve outcome, both in operation room and ICU patients.
The findings of the meta-analysis seem in contrast, at least in part with findings in previous studies reporting clinical benefit of chloride-poor or balanced solutions versus isotonic saline in operation room and ICU patients. One retrospective observational study in operation room patients undergoing major surgery evaluated the effect of balanced versus non-balanced fluid showed no differences in mortality, but there was a 5-fold increase in the need for RRT in patients resuscitated with isotonic saline (33). This study, however, had several limitations, including differences in matched cohort baseline characteristics and increased use of balanced fluids in teaching hospitals possibly indicating differences in standard of care. A prospective observational study in 1,500 ICU patients also did not show an association between use of balanced solutions and mortality, but did find a marked decrease of AKI with use of balanced solutions and need for RRT (34). In this study, use of colloids was not excluded, which could have affected outcomes. One retrospective observational study found an association between use of balanced fluids and in-hospital death in ICU patients with sepsis, though no differences in the occurrence of renal failure or need for RRT (35). Notably, in this study less than 5% of patients actually received balanced solutions, and over 20% of patients received colloids.
Fluid resuscitation with balanced solutions could come with important side-effects. All balanced solutions to date contain buffering agents, like lactate, acetate, citrate or malate. These all are converted in the liver. Over-use of these buffers might result in metabolic alkalosis, or in the setting of either liver failure or shock, conversion could be severely impaired (11), e.g., leading to hyperlactatemia. Other side effects include increased nitric oxide production due to acetate, which could lead to hypotension and cardiac dysfunction (36). Finally, some balanced solutions contain magnesium which can induce bradycardia and increase peripheral vascular resistance leading to decrease in microcirculation and worsening of organ ischemia (37). Most of these side-effects were not reported in the trials analyzed, which is not surprising as they were not systematically looked for.
This systematic review shows that the number of high quality RCTs published on this topic is low, which is surprising seen the fact that these fluids are massively described and used in daily clinical practice worldwide. The operation room and ICU communities need to perform well-powered studies, preferably well-designed RCTs, which not only focus on patient-centered endpoints such as mortality, length of stay in ICU and hospital, development of AKI and need for RRT, but also the potential side-effects summarized above.
Our meta-analysis also knows several limitations. First of all, the analysis was limited due to the low number of RCTs that fulfilled all in- and exclusion criteria. The fact that practically all outcomes were only reported by some eligible trials is another limitation. Indeed, unreported outcomes could lead to overestimation of effects in meta-analyses (38). Secondly, five out of eight RCTs in the operation room were performed in patients undergoing renal transplantation who are high risk for development of dilutional acidosis. Furthermore, we were limited by the fact that the kidney injury scoring was not reported in seven RCTs and could not be used in two out of the four remaining RCTs because they were performed before the current scoring system was implemented. Need for RRT was easier to capture, however, but we may have had insufficient power to find a difference. Also, the results of the meta-analysis were highly influenced by one single large RCT (22). Finally, the incidence of clinical relevant outcomes, as used in this meta-analysis was very low in operation room patients, reducing its power.
Conclusions
The present meta-analysis does not support the use of balanced solutions for fluid resuscitation, neither in ICU nor in operation room patients.
Search strategy
1. randomized controlled trial [ptyp]
2. controlled clinical trial [ptyp]
3. randomized [Title/Abstract]
4. placebo [Title/Abstract]
5. drug therapy [Subheading]
6. randomly [Title/Abstract]
7. trial [Title/Abstract]
8. groups [Title/Abstract]
9. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
10. animals [mh] NOT humans [mh]
11. #9 NOT #10
12. (fluid resuscitation)[Title/Abstract] OR fluid therapy[Title/Abstract])
13. ("crystalloid solutions" [Supplementary Concept]) OR ("Isotonic Solutions"[Mesh])
14. ("Balance*"[Title/Abstract] OR "buffer*"[Title/Abstract]) AND ("saline"[Title/Abstract] OR "solution*"[Title/Abstract] OR "crystalloid*"[Title/Abstract] OR "Fluid*"[Title/Abstract])
15. (“chloride*”[Title/Abstract]) AND (“content”[Title/Abstract] OR “poor”[Title/Abstract] OR “rich”[Title/Abstract] OR “high”[Title/Abstract] OR “low”[Title/Abstract] OR “liberal”[Title/Abstract] OR “restrict*”[Title/Abstract])
16. (“Plasmalyt*”[Title/Abstract] OR “Plasma-lyte” [Title/Abstract])
17. “Sterofundin” [Title/Abstract] OR “Ringerfundin” [Title/Abstract] OR “isofundin” [Title/Abstract]
(“Ringer*”[Title/Abstract] AND (“solution*”[Title/Abstract] OR "Lactate*"[Title/Abstract] OR “Acetate*”[Title/Abstract]))
(“Hartmann*”[Title/Abstract] AND (“solution*” [Title/Abstract])
#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19
#11 AND #20
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med 2013;39:165-228. [Crossref] [PubMed]
- McIntyre LA, Hébert PC, Fergusson D, et al. A survey of Canadian intensivists' resuscitation practices in early septic shock. Crit Care 2007;11:R74. [Crossref] [PubMed]
- Yunos NM, Bellomo R, Story D, et al. Bench-to-bedside review: Chloride in critical illness. Crit Care 2010;14:226. [Crossref] [PubMed]
- Handy JM, Soni N. Physiological effects of hyperchloraemia and acidosis. Br J Anaesth 2008;101:141-50. [Crossref] [PubMed]
- Guidet B, Soni N, Della Rocca G, et al. A balanced view of balanced solutions. Crit Care 2010;14:325. [Crossref] [PubMed]
- Kellum JA, Song M, Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest 2006;130:962-7. [Crossref] [PubMed]
- Kellum JA, Song M, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest 2004;125:243-8. [Crossref] [PubMed]
- Wilkes NJ, Woolf R, Mutch M, et al. The effects of balanced versus saline-based hetastarch and crystalloid solutions on acid-base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesth Analg 2001;93:811-6. [Crossref] [PubMed]
- George AK, Shih A, Regan TJ. Effect of acute ketoacidosis on the myocardium in diabetes. Am J Med Sci 1996;311:61-4. [Crossref] [PubMed]
- Morgan GE, Mikhail MS, Murray MJ. Clinical anesthesiology. 4th ed. New York: Lange Medical Books/McGraw Hill Medical Pub. Division, 2006:1105.
- Veech RL. The toxic impact of parenteral solutions on the metabolism of cells: a hypothesis for physiological parenteral therapy. Am J Clin Nutr 1986;44:519-51. [PubMed]
- McFarlane C, Lee A. A comparison of Plasmalyte 148 and 0.9% saline for intra-operative fluid replacement. Anaesthesia 1994;49:779-81. [Crossref] [PubMed]
- Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 1999;90:1265-70. [Crossref] [PubMed]
- Waters JH, Gottlieb A, Schoenwald P, et al. Normal saline versus lactated Ringer's solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesth Analg 2001;93:817-22. [Crossref] [PubMed]
- O'Malley CM, Frumento RJ, Hardy MA, et al. A randomized, double-blind comparison of lactated Ringer's solution and 0.9% NaCl during renal transplantation. Anesth Analg 2005;100:1518-24. [Crossref] [PubMed]
- Hadimioglu N, Saadawy I, Saglam T, et al. The effect of different crystalloid solutions on acid-base balance and early kidney function after kidney transplantation. Anesth Analg 2008;107:264-9. [Crossref] [PubMed]
- Khajavi MR, Etezadi F, Moharari RS, et al. Effects of normal saline vs. lactated ringer's during renal transplantation. Ren Fail 2008;30:535-9. [Crossref] [PubMed]
- Wu BU, Hwang JQ, Gardner TH, et al. Lactated Ringer's solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin Gastroenterol Hepatol 2011;9:710-7. [Crossref] [PubMed]
- Kim SY, Huh KH, Lee JR, et al. Comparison of the effects of normal saline versus Plasmalyte on acid-base balance during living donor kidney transplantation using the Stewart and base excess methods. Transplant Proc 2013;45:2191-6. [Crossref] [PubMed]
- Potura E, Lindner G, Biesenbach P, et al. An acetate-buffered balanced crystalloid versus 0.9% saline in patients with end-stage renal disease undergoing cadaveric renal transplantation: a prospective randomized controlled trial. Anesth Analg 2015;120:123-9. [Crossref] [PubMed]
- Young JB, Utter GH, Schermer CR, et al. Saline versus Plasma-Lyte A in initial resuscitation of trauma patients: a randomized trial. Ann Surg 2014;259:255-62. [Crossref] [PubMed]
- Young P, Bailey M, Beasley R, et al. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA 2015;314:1701-10. [Crossref] [PubMed]
- Krajewski ML, Raghunathan K, Paluszkiewicz SM, et al. Meta-analysis of high- versus low-chloride content in perioperative and critical care fluid resuscitation. Br J Surg 2015;102:24-36. [Crossref] [PubMed]
- Burdett E, Dushianthan A, Bennett-Guerrero E, et al. Perioperative buffered versus non-buffered fluid administration for surgery in adults. Cochrane Database Syst Rev 2012;12:CD004089. [PubMed]
- Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev 2013;2:CD000567. [PubMed]
- Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0.9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg 2012;256:18-24. [Crossref] [PubMed]
- Reid F, Lobo DN, Williams RN, et al. (Ab)normal saline and physiological Hartmann's solution: a randomized double-blind crossover study. Clin Sci (Lond) 2003;104:17-24. [PubMed]
- McCluskey SA, Karkouti K, Wijeysundera D, et al. Hyperchloremia after noncardiac surgery is independently associated with increased morbidity and mortality: a propensity-matched cohort study. Anesth Analg 2013;117:412-21. [Crossref] [PubMed]
- Martin G, Bennett-Guerrero E, Wakeling H, et al. A prospective, randomized comparison of thromboelastographic coagulation profile in patients receiving lactated Ringer's solution, 6% hetastarch in a balanced-saline vehicle, or 6% hetastarch in saline during major surgery. J Cardiothorac Vasc Anesth 2002;16:441-6. [Crossref] [PubMed]
- Dubin A, Pozo MO, Casabella CA, et al. Comparison of 6% hydroxyethyl starch 130/0.4 and saline solution for resuscitation of the microcirculation during the early goal-directed therapy of septic patients. J Crit Care 2010;25:659. [Crossref] [PubMed]
- Bayer O, Reinhart K, Kohl M, et al. Effects of fluid resuscitation with synthetic colloids or crystalloids alone on shock reversal, fluid balance, and patient outcomes in patients with severe sepsis: a prospective sequential analysis. Crit Care Med 2012;40:2543-51. [Crossref] [PubMed]
- Perner A, Haase N, Guttormsen AB, et al. Hydroxyethyl starch 130/0.42 versus Ringer's acetate in severe sepsis. N Engl J Med 2012;367:124-34. [Crossref] [PubMed]
- Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0.9% saline compared to Plasma-Lyte. Ann Surg 2012;255:821-9. [Crossref] [PubMed]
- Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA 2012;308:1566-72. [Crossref] [PubMed]
- Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis*. Crit Care Med 2014;42:1585-91. [Crossref] [PubMed]
- Tiranathanagul K, Tangvoraphonkchai K, Srisawat N, et al. Acute intradialytic cardiac function and inflammatory cytokine changes during high-efficiency online hemodiafiltration with acetate-free and standard dialysis solutions. Ther Apher Dial 2015;19:250-8. [Crossref] [PubMed]
- Rizoli S. PlasmaLyte. J Trauma 2011;70:S17-8. [Crossref] [PubMed]
- Furukawa TA, Watanabe N, Omori IM, et al. Association between unreported outcomes and effect size estimates in Cochrane meta-analyses. JAMA 2007;297:468-70. [Crossref] [PubMed]