The role of reactive oxygen species in the pathophysiology of cardiovascular diseases and the clinical significance of myocardial redox
Introduction
Cardiovascular diseases including coronary artery disease, hypertensive heart diseases, and stroke are the leading cause of death worldwide (1). Recent studies have reported that overproduction of oxidative stress-related factors like reactive oxygen species (ROS) can cause myocardial infarction, atherosclerosis, and diabetes (2).
ROS participate in cell signaling as mediators and regulators of vascular function. ROS include free radicals such as superoxide anion (O2–), lipid radicals (ROO–), hydroxyl radical (HO–), nitric oxide (NO) and not free radicals such as hydrogen peroxide (H2O2), hypochlorous acid (HClO) and peroxynitrite (ONOO–), that have oxidizing effects and contribute to oxidative stress (2).
ROS are produced by all vascular layers, including endothelium, smooth muscle, and adventitia (2). Endothelium has an essential role in the regulation of vascular tone, modulation of inflammation, and promotion or inhibition of vascular growth and platelet aggregation and coagulation (1,2). Under physiological conditions, ROS act as signaling molecules that regulate vascular smooth muscle cell contraction, relaxation, and growth (1). Pathophysiological conditions induce an imbalance between ROS (oxidants) and antioxidants, that plays a significant role in endothelial dysfunction and various cardiovascular disease conditions (1,2). ROS have been implicated in cell damage, necrosis and cell apoptosis due to their direct oxidizing effects on macromolecules like proteins, lipids and DNA (2).
Abnormal production of free radicals leads to increased oxidative stress on cellular structures and causes changes in molecular pathways that underpin the pathogenesis of cardiovascular diseases (2).
The role of ROS in cardiac hypertrophy
Cardiac hypertrophy could normally occur in response to increases in pressure and volume overload, such as strenuous exercise. Therefore, hypertrophy could be the result of sustained hemodynamic load associated with hypertension, MI, aortic or mitral valve regurgitation, or neurohormones. Hypertrophic myocyte is characterized by a transformed structure and function, induction of hypertrophy-linked genes such as c-fos, c-jun, and erg-1, as well as increased protein synthesis. Numerous extracellular stimuli, including AngII, ET-1, TNF-a, could induce the hypertrophy of myocytes, via eliciting various downstream signaling pathways. Signal transduction factors include MAP kinases, PKC, NFkB, calcineurin, and tyrosine kinases. ROS directly or indirectly activate these pathways, playing a key role in cardiac hypertrophy.
Angiotensin-II-induced cardiac hypertrophy is a well-studied model of hypertrophy induced by an extracellular signal. Ang-II hypertrophic effects are mainly mediated by the G-coupled receptor AT1R. After its binding to the receptor, Ang-II induces the production of ROS, which subsequently leads to the activation of various, mainly ROS-regulated signaling kinases. The expression of the hypertrophic factor beta-myosin heavy chain in myocytes stimulated by Ang-II is mediated by ROS-dependent activation of the ERK kinase signaling cascade (3). In a guinea pig model of progressive left ventricular hypertrophy, expression of p22phox, p67phox, p47phox, Nox2, and Nox activity were up-regulated during the progression of cardiac hypertrophy to failure (4). In addition, Ang-II could stimulate NADPH oxidase to produce ROS, leading to p38MAP kinase activation and the subsequent activation of AP-1, while it has also been demonstrated that Rac1 activation seems to be a critical step in the development of hypertrophy (5). Data also indicated that Rac1 activation is followed by ASK-1 and NFkB activation (6). Furthermore, deletion of ASK-1 attenuates the expression of p38 and JNK, resulting in reduced hypertrophic effects (7).
However, there are also recent experimental data, emerging from the use of transgenic mice models, proposing that ASK-1 does not directly mediate the hypertrophic pathophysiological changes, but probably increases the propensity of the cardiomyocytes to develop a hypertrophic phenotype, a mechanism mediated by JNK activation but not ERK (8). Recent data have shown the possible direct association between ROS production and the activation of growth factors which mediate the hypertrophic response. Moreover, AngII potentiates Nox2 activation and increases ROS production, which in turn stimulates the expression of WNT1 inducible signaling pathway protein 1, which is a well-known growth factor (9).
ROS in cardiac fibrosis and contractile dysfunction
Cardiac fibrosis is characterized by increased collagen production from activated cardiac fibroblasts. It is among the most important functional and structural changes occurring in various heart diseases. These changes could be mainly attributed to defects in biochemical mechanisms, or to alterations in myocyte contractile system, or usually both. As it has been previously demonstrated in animal models, pressure overload could lead to increased interstitial fibrosis, described as increased expression of procollagen I and III at the molecular level, and significant contractile dysfunction, alterations closely associated with increased NADPH oxidase activation (mainly Nox2) (8). The same study also demonstrated that Nox2 activation does not seem to be connected with the development of cardiac hypertrophy (10). Apart from the indirect effect of ROS on cardiac contractility, recent data indicated that ROS could directly affect the function of the heart contractile function. It has been reported that ROS could directly enhance the positive inotropic effect of agents such as ET-1 possibly by activation of redox signaling pathways such as ERK1/2 (11). It has also been described that ROS reduces the positive inotropic effect of an agent. In particular, it has been demonstrated that inhibition of ROS production, via inhibiting NADPH oxidase activation, resulted in improved phosphorylation status of phospholamban, and enhanced positive inotropic effect after treatment with dobutamine (12). Moreover, in a model of heart failure, it has been demonstrated that the activation of MAPK, specifically of p38 MAP kinase, may be an initial step that could lead or it is closely connected to ROS overproduction (13). Thus, ROS could oxidize the myofibrillar proteins, resulting in contractile dysfunction observed in heart failure (14).
The role of myocardial redox in various clinical states
ROS in myocardial infarction and ischemia-reperfusion injury
ROS contribute to the genesis and the progression of the coronary artery disease. The formation of oxidized LDL, an initial step in atherosclerosis progression, is mediated by ROS. Moreover, ROS could also lead to matrix metalloproteinases activation, resulting in plaque rupture. However, ROS also seem to play a major role in the setting of acute MI, as well as following reperfusion therapy.
Myocardial redox state is positively associated with the extent of necrosis after MI as well as with reperfusion injury. Initial studies which exploited experimental mice models that overexpress SOD, showed better post-ischemic cardiac function compared to control mice, indicating the significant role of ROS. However, studies are demonstrating that SOD alone might not offer sufficient protection. On the other hand, it seems that ROS production favors the mechanisms of late preconditioning, which is the endogenous myocardial defense mechanism against reperfusion injury, an action possibly mediated by NFkB activation (15).
Several mechanisms have been proposed for ROS production in myocardial tissue after MI. Initial studies indicated XOR as the potential source of ROS in reperfused tissue. In a state of ischemia, XDH is converted to the oxidized form, leading to superoxide formation (16). It has been proposed that in ischemia ATP is in excess, leading to elevated Ca2+ levels. This could lead to Ca2+ dependent proteases which convert XDH to XO. In addition, the use of allopurinol, an established XO inhibitor, has led to reduced ROS production and improved myocardial function after MI (17). Moreover, experimental data have also shown that there is increased expression of Nox2 in cardiomyocytes after MI (18). The increased expression of Nox2 was mainly observed in the area of MI, though it is also present in cardiomyocytes away from the infarcted area (18). This could also be an explanation for the actual involvement of Nox2, mainly via ROS production and induction of redox-regulated pathways, in the cardiac remodeling observed after MI (19). Furthermore, Nox2 seem to play a crucial role in preconditioning during the early ischemic phase. Recent studies have demonstrated that gp91phox expression was significantly increased in the infarcted myocardium of mice, particularly in the early stage of MI (20). Monocyte-derived macrophages were found to be the major inflammatory cells in the infarcted myocardium, mainly characterized by increased expression of gp91phox (20). The expression pattern of macrophages gp91phox is similar to the expression of NADPH in overall myocardial tissue, with a declining phase 2 weeks after MI, thus indicating macrophages as a dominant source of ROS in infracted myocardium (20). Additional data also showed that lack of p47phox subunit, could improve cardiac function and survival in p47phox knockout mice, after experimentally induced MI (21). Recent data demonstrated improved cardiac function in MI animal models, after molecular silencing of cardiomyocyte Nox4 (22). Nox4 seem to play an important role in MI-induced cardiomyocyte apoptosis and overactivation of the sympathetic nervous system, both important mechanisms mediating the development of heart failure (22). Conclusively, NADPH oxidase is a major source of ROS in infarcted myocardium and could serve as a possible target for molecular and pharmaceutical therapies. Furthermore, there are also data indicating that ROS production could directly affect the activation of signaling pathways regulating pro-survival and pro-apoptotic signals. It has been demonstrated that ROS, or more correctly the redox imbalance observed in rat MI model, are related to a decrease in Akt and mTOR phosphorylation, supposing that this could also contribute to molecular and structural abnormalities observed after MI (14).
Apart from the mechanisms mediating coronary artery occlusion, ROS play a vital role in the injury following MI. Although reperfusion of the ischemic myocardium during early stages is essential in preventing cardiac damage, reperfusion of the ischemic heart, after a certain critical period, exerts deleterious effects, a phenomenon also known as ischemia/reperfusion (I/R) injury.
Myocardial I/R changes the metabolic status of the cardiomyocyte resulting in concomitant alterations of its redox status. More specifically, during ischemia the electron transport chain is in a reduced state, whereas during reperfusion their interaction with molecular oxygen leads to ROS formation, being the key mechanism for myocardial damage. Oxidative stress in I/R hearts is associated with cardiac dysfunction, impaired antioxidant enzyme activity, and increased lipid peroxidation, facilitating membrane permeability and resulting in the generation of unsaturated aldehydes; thus, it affects the activation status of various molecular components and elicits various signaling pathways. For example, recent data demonstrated that ROS could induce the generation of 4-hyroxy-2-nonenal, an unsaturated aldehyde, which in turn induces the activation and the nuclear translocation of the Nf-E2-related factor (Nrf2), which seems to mediate protective actions for the myocardium, mainly by favoring the activation status of antioxidant enzymes (23).
Cell death occurring during I/R injury is characterized by the accumulation of oxidized cellular lipids and proteins due to oxidative stress. It is a result of the formation of mitochondrial ROS, closely associated with dysfunctional mitochondrial Ca2+ channels, and activation of redox-sensitive factors, such as ERK and JNK (24). The activation of these signaling pathways could further enhance the dysfunction of mitochondrial oxidases, leading to further ROS production, as well as the Ca2+ dyshomeostasis, which is key factors leading to cardiomyocyte death and, I/R induced myocardial injury (25). However, it seems that molecular mechanisms linking I/R and ROS are more complex. Various experimental studies have indicated the contribution of ROS in the induction of cardiomyocyte protecting mechanisms. Recent data demonstrated that ROS levels are closely connected to the expression of various molecular factors, such as macrophage migration, inhibitory factors or FoxO transcription factor and others. These factors could potentiate the activation of cell survival pathways, resulting in a better myocardial response to the I/R injury, possibly via improving the myocardial redox status (26,27). C-Jun, a prominent member of AP-1 transcription factors family, also seem to act in the background, mainly orchestrating the inflammatory part of I/R myocardial injury. The suppression of c-Jun expression was shown to significantly reduce the oxidative stress in the reperfused myocardium and the expression of factors such as matrix metalloproteinases, leading to reduced damage (28). Moreover, it was recently proven that reduced L-arginine availability is a fundamental element in the pathogenesis of I/R injury. Increasing L-arginine availability improves myocardial responses to I/R (29). This could be an indication of possible participation of nitric oxide synthase (NOS) in the pathogenesis of I/R injury. Furthermore, a recent study demonstrated that improvement of iNOS coupling, via BH4 restoration, has led to increase NO bioavailability and reduced infarct size (30). This effect is mainly mediated via enhanced protein S-nitrosylation, a newly emerged pathway by which NOS may mediate its actions (30). Recently, a new mechanism mediating ROS actions in I/R injury was investigated, demonstrating cysteines of protein tyrosine phosphatases, as possible targets for ROS. The oxidation of these phosphatases by ROS seem to lead to induction of redox-regulated signaling pathways, such as tyrosine kinase signaling pathways (31).
ROS and heart failure
Experimental and clinical data indicate an increase in myocardial redox state along with the development of heart failure. Oxidative stress seems to have a key role during the transition from compensated hypertrophy to heart failure (32). Furthermore, activation of ROS sources in heart failure has also been described. There is increased activity of NADPH oxidase, while mitochondrial oxidases have been highlighted as a source of ROS in heart failure. Furthermore, in vivo and in vitro data revealed a reduced antioxidant capacity, as it is demonstrated by reduced activity of antioxidant enzymes such as SOD and GPx.
One of the most important physiological mechanisms affected in the context of heart failure is the excitation-contraction coupling of the cardiomyocytes, leading to motion abnormalities of the cardiac muscle. Interestingly, reduced Ca2+ levels in the SR of failing cardiomyocytes has been observed, given that Ca2+ level is the main regulator of the proper function of the cardiomyocyte. Recent data have shown that ROS directly affect the function of RyR, mainly by oxidizing cysteine thiols, leading to conformational changes and resulting in increased Ca2+ release by SR (33). This phenomenon could be attributed to an increased sensitivity of RyR in heart failure, which could lead to abnormal activation and Ca2+ leakage (33). Additional data has also demonstrated mitochondrial-deriving ROS as a dominant player in the development of heart failure, thus proposing the design of mitochondria-targeted drugs (34).
Furthermore, ERK signaling has been suggested as one of the responsible pathways involved in the progression from cardiac hypertrophy to heart failure (14). Additionally, the source of oxidant and antioxidant enzyme activities in the right ventricle (RV) and left ventricle (LV) of human failing hearts were investigated, and a significant increase in superoxide production especially by NADPH oxidase in both failing ventricles was found (35). However, a higher ROS production was observed in RV, which is also accompanied by higher malondialdehyde levels, an index of lipid peroxidation. CAT and GPx activities were increased and positively correlated with the increase in NADPH oxidase-dependent superoxide production (35). However, RV exhibited a slower response of the antioxidant defense system. This difference seems to be also correlated with a generalized redox status of RV compared to LV, as well as increased activation of redox-sensitive pathways (36). On the other hand, other studies underlined that the activity of antioxidant enzymes could not change under circumstances of increased ROS levels in heart failure or even a decreased activity. These discrepancies can be attributed to a possible differential regulation of mRNA/protein expression, which may attribute to the phosphorylation status of various molecular components, i.e., the phosphorylation of tyrosine of the enzymes, or it may reflect a more complicated regulatory mechanism (35).
ROS and atrial fibrillation
It is generally accepted that myocardial redox state affects the electrophysiological function of the heart. Previous and recent experimental data tried to investigate this association in the context of atrial fibrillation, which is one of the most common arrhythmias in clinical practice. Patients with atrial fibrillation present higher circulating levels of inflammatory markers and markers of oxidative stress, which seem to associate with the pathogenesis of AF (37) directly. However, the exact mechanisms connecting the generation of ROS with the development or the evolution of the atrial fibrillation remain relatively unidentified. One of the most interesting findings was that atrial NADPH oxidase activity is independently associated with an increased risk of postoperative AF (38). Further data demonstrated higher NADPH-stimulated O2– production in atrium homogenates derived from AF patients compared to patients with sinus rhythm (39). According to the observations of this study, Nox2 is the primary NADPH oxidase that serves as a source of ROS in fibrillating atrial cardiomyocytes (39). The same study also showed that NOS uncoupling was significantly higher in the myocardium of patients with AF, indicating NOS as another source of ROS in the fibrillating myocardium (39). Experimental studies showed that atrial mRNA expression of p22phox, p67phox, and rac1 was enhanced by Ang-II infusion (40). Rac1 activation is an upstream event in the production of ROS by renin-angiotensin system activation (40). According to an immunohistochemical study, human atrial myocytes can significantly produce superoxide through a membrane-bound nox2 NADPH oxidase (41). Beyond NADPH oxidase, an increased activity of XO enzymatic system in the fibrillating atrium has also been observed (42). Furthermore, numerous studies tried to investigate the role of NOS in atrial fibrillation, though existing data are difficult to interpret. It seems that NOS expression may be affected in atrial fibrillation. It has been proposed that probably iNOS expression levels is merely associated with cell damage and apoptosis observed in atrial fibrillation (43). Recent data from immunohistochemical analyses showed that possibly eNOS myocardial expression is not directly linked to atrial fibrillation pathophysiological processes (44). Furthermore, in animal models of rapid atrial pacing, a significant down-regulation of ventricular eNOS expression was described, indicating a possible contribution of eNOS in tachyarrhythmia-induced injury. However, in order to elucidate the association between ROS and atrial fibrillation, further studies are needed, combining both experimental and clinical data.
ROS and diabetes mellitus (DM)
Oxidative stress plays a detrimental role in the development of vascular complications in patients with DM. Free radical accumulation in the vasculature of diabetic patients triggers vessel inflammation and ROS generation (45).
Free fatty acid accumulation impairs Akt activity and phosphorylation of eNOS, resulting in decreased production of NO, endothelial dysfunction and vascular remodeling. In turn, accumulation of ROS activates transcription factor NF-kB, leading to increased expression of inflammatory adhesion molecules and cytokines. Growing evidence suggests that hyperglycemia decreases endothelium-derived NO availability and affects vasomotion via overproduction of ROS. Hyperglycemia targets the mitochondrial electron transport chain leading to a net increase in O2– formation. Moreover, ROS-induced activation of protein kinase C (PKC) further increases O2– formation. Activation of PKC leads to up-regulation of NADPH oxidase, thromboxane production and impaired NO release. Mitochondrial ROS trigger inflammatory cascades involved in the pathogenesis of cardiovascular complications, including polyol flux, advanced glycation end-products (AGEs) and their receptors (RAGEs) and PKC. Hyperglycemia-induced ROS formation is associated with the persistence of vascular dysfunction irrespective of glucose levels normalization. This phenomenon of ‘metabolic memory’ along with ROS-driven epigenetic changes, unveils the pathophysiology of macro- and microvascular disease progress in DM, despite intensive glycemic control. Finally, ROS-driven pathways affect the coronary circulation, lead to myocardial hypertrophy and fibrosis and therefore set the substrate of diabetic cardiomyopathy (45).
Common drug classes and myocardial redox
The data presented above suggest that oxidative stress is one of the most common underlying mechanisms provoking myocardial diseases. The need to study the effects of commonly used drugs on myocardial redox has become crucial. The formation of new therapeutic strategies based on targeting myocardial redox becomes of utmost importance.
Statins
Statins are one of the most common used lipid-lowering agents, acting by inhibiting hydroxyl-methyl-coenzyme A reductase. Thus, statins inhibit cholesterol biosynthesis pathway. However, constantly emerging data indicate a pleiotropic role for statins. Although the antioxidant effect of statins is predominantly associated with vascular wall, statins exert a beneficial effect on the myocardium through multifactorial pathways.
Clinical studies have shown that statins could have a positive clinical impact on various diseases of human myocardium such as cardiac hypertrophy, heart failure, and cardiomyopathies. One of the first observations was that oral statin treatment has led to decreased Rac1 activity in the human myocardium from patients with heart failure. This subsequently has also resulted in decreased NADPH oxidase-depended ROS production (46). Furthermore, this could also have a substantial clinical impact. Treatment of cardiomyocytes with simvastatin led to decreased TNF-a production, mainly due to decreased NADPH-oxidase expression and decreased ROS production (47). Moreover, in vitro treatment of cardiomyocytes with rosuvastatin attenuated AngII-mediated cardiomyocyte growth and oxidative stress (11). This was mediated by reduced NADPH oxidase and NFkB expression, indicating a direct effect of statin on intracellular redox signaling pathways (11). Furthermore, recent in vitro data underpinned that treatment of cardiomyocytes with statins seem to protect cardiomyocyte from ROS deleterious effect also via maintaining the mitochondrial membrane stability, mainly through regulating the function of mitochondrial ion channels (48). Moreover, statin therapy also affects the expression of receptors closely associated with ROS production such as the receptor of oxidized LDL (11,49).
Statins have also been shown to affect the expression of NOS. Initial experimental observations indicate that statins could exert its beneficial effects, especially in cases of myocardial infarction and ischemia/reperfusion injury, by affecting NOS expression. Treatment with rosuvastatin has led to decreased I/R injury possibly via increasing eNOS expression and reducing iNOS expression, in a rat model (50). It has also been suggested that statins could be used as a treatment for atrial fibrillation, especially as an upstream therapy before by-pass surgery or invasive procedures (51). However, further clinical studies, as well as in vitro studies are necessary in order to clarify the clinical efficacy of statins as “antiarrhythmic” drugs.
Calcium channel blockers
Calcium channel blockers act by blocking the Ca exchange via calcium channels thus regulating the contraction of the heart. Various experimental data demonstrated the beneficial role of this drug class in different heart diseases. In vitro data implicated the activation of pro-survival pathways as a mechanism mediating the cardioprotective effects of calcium blockers (52). Treatment with verapamil improved the redox state in a model of ischemia-reperfusion injury. Furthermore, verapamil induced the activation of Akt and ERK1/2 pathways, leading to a number of cardioprotective effects, such as the downregulation of apoptotic pathways (52). It was also proposed that this may be a mechanism by which calcium blockers amplify the ROS-induced damage, though a direct association between ROS and cardioprotective mechanisms of calcium channels blockers is not clearly demonstrated (52). In addition, data indicate that amlodipine could have a beneficial effect on hypertension-induced cardiac hypertrophy by reducing the activity of NADPH oxidase (53). Amlodipine, as well as the combination of amlodipine plus atorvastatin, resulted in a significant reduction of p47phox and p40phox, p22phox and Rac1 expression levels in heart tissue from spontaneously hypertensive rats, leading to decreased interstitial fibrosis and cardiomyocyte size, probably by suppressing ROS generation and subsequent activation of redox-activated signaling pathways (53). Apart from its effect on ROS NADPH oxidase, calcium blockers seem to favor the activity of antioxidant enzymes. Data from spontaneously hypertensive rats indicated that treatment with calcium blockers could lead to increased activation of Cu/Zn SOD in the heart, suggesting an alternative mechanism for calcium blockers antioxidant properties (54).
Antioxidants
Numerous studies have tried to investigate the possible beneficial role of antioxidants in various heart disease states, via suppressing the production of ROS. Various hypothetical mechanisms of action have been proposed. Crocetin, a natural carotenoid, has been shown to protect the in vitro Ang-II-induced hypertrophic transformation of cardiomyocytes and cardiac fibroblasts by inhibiting the ROS-dependent activation of the ERK1/2 inflammatory signaling pathway. Polyphenols are considered molecular compounds that have anti-inflammatory and antioxidant properties. Resveratrol is a polyphenol which is usually present in red wine. The actions of resveratrol on heart physiology have been extensively studied. Numerous studies have shown that resveratrol could improve the myocardial redox state. This compound could improve the viability of cardiomyocyte via the activation of AMP kinase (55). It has also been demonstrated that resveratrol could enhance the activation of antioxidant defense system, but also to increase the bioavailability of myocardial NO (56). Treatment with proanthocyanidins, a natural antioxidant, has also been described to improve the function of the cardiomyocyte. It has been demonstrated that treatment with grape seed proanthocyanidins could have cardioprotective effects via improving NO bioavailability and decreasing ROS production in a model of I/R injury (57). These actions have been proposed to be mediated via Akt-NOS signaling pathway (57). Similar results were also described in a model of doxorubicin-induced cardiotoxicity (58). Grape seed proanthocyanidins have sufficiently decreased the superoxide, and HO– generation induced by doxorubicin treatment and improved the antioxidant capacity of cardiomyocytes (59).
Other therapies
As previously mentioned xanthine oxidase is among the key sources of ROS in human cardiomyocytes. Several experimental studies tried to investigate the possible favorable effect of XOR inhibition on the myocardial redox state (59). It has been indicated that allopurinol, which acts via XOR inhibition, could directly affect the myocardial ROS levels, by reducing the activation status of XOR, as well as by affecting the expression of iNOS and NADPH oxidase (59). As we have also previously described BH4, as a crucial NOS cofactor, holds a key role both on vascular wall homeostasis, as well as on myocardial function. However, it remains unclear if BH4 exogenous restoration could exert a beneficial effect (60). Animal studies have described that treatment with BH4 led to improved eNOS coupling, as well as decreased ROS levels, in the heart of rats subjected to coronary artery ligation (61). It has also been described that rats receiving BH4 had a better cardiac phenotype compared to the non-treated group (61). Same results were recently described regarding the diastolic cardiac function of mice subjected to various experimental methods which have led to cardiac hypertrophy and oxidative modifications in the myocardial tissue (62). It has been shown that uncoupled NOS seems to significantly contribute to the ROS-induced functional changes of the hypertensive heart muscle. Short term BH4 treatment, restored the BH4 levels leading to improved cardiac function, suggesting that BH4 could be a potent therapeutic target (62). However, more studies are needed to define the exact mechanism of BH4 regulation (63). It has been shown that pretreatment of rats with high doses of folic acid has led to decreased superoxide production after 30min coronary artery occlusion, as well as improved eNOS coupling and reduced size of the infarcted area (64).
Recently, Bubb et al. demonstrated that the bardoxolone derivative DH404 significantly attenuated cardiac remodeling post MI by re-coupling of eNOS (65). In the same frame, Apurinic/apyrimidinic endonuclease/redox factor 1 (APE1) seems to attenuate stem cell apoptosis in MI model that in turn resulted in less post-infarct fibrosis and improved cardiac function via NF-kB pathway activation (66).
Table S1 summarizes the studies investigating ROS sources, signaling pathways, clinical implications and antioxidant efficacy.
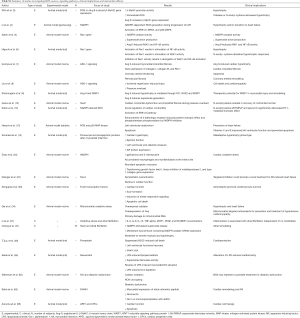
Full table
Conclusions
Accumulated evidence from clinical and experimental animal studies supports a decisive role for redox signaling in cardiovascular homeostasis and disease. ROS play a crucial role in the development and evolution of heart disease. Cardiovascular disease mechanisms are strongly linked to the production of ROS and the dysregulation of endogenous oxidant-antioxidants pathways.
Unveiling the molecular mechanisms of disease pathogenesis and progression is essential in providing suitable targets in order to develop innovative therapeutic strategies. These therapeutic strategies can reduce ROS production or enhance ROS degradation resulting in protective effects against cardiovascular diseases.
A clear understanding of the exact role of ROS in the pathophysiology of heart disease and how ROS regulate signaling pathways can play a pivotal role in the pathogenesis and disease progression. It can allow a better understanding of how ROS may cause or contribute to disease and uncover novel treatments, such as antioxidant gene therapy and nanotechnology-related antioxidant delivery.
Oxidative stress remains an attractive target for cardiovascular prevention and therapy. Further experimental investigation and large-scale prospective randomized trials should be designed to evaluate the role of ROS in cardiovascular pathology.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kim H, Yun J, Kwon SM. Therapeutic Strategies for Oxidative Stress-Related Cardiovascular Diseases: Removal of Excess Reactive Oxygen Species in Adult Stem Cells. Oxid Med Cell Longev 2016;2016:2483163.
- Ray PD, Huang PW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 2012;24:981-90. [Crossref] [PubMed]
- Shih NL, Chang T, Loh S, et al. Reactive oxygen species modulate angiotensin II-induced beta-myosin heavy chain gene expression via Ras/Raf/extracellular signal-regulated kinase pathway in neonatal rat cardiomyocytes. Biochem Biophys Res Commun 2001;283:143-8. [Crossref] [PubMed]
- Li JM, Gall NP, Grieve DJ, et al. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension 2002;40:477-84. [Crossref] [PubMed]
- Satoh M, Ogita H, Takeshita K, et al. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA 2006;103:7432-7. [Crossref] [PubMed]
- Higuchi Y, Otsu K, Nishida K, et al. The small GTP-binding protein Rac1 induces cardiac myocyte hypertrophy through the activation of apoptosis signal-regulating kinase 1 and nuclear factor-kappa B. J Biol Chem 2003;278:20770-7. [Crossref] [PubMed]
- Izumiya Y, Kim S, Izumi Y, et al. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodelling. Circ Res 2003;93:874-83. [Crossref] [PubMed]
- Liu Q, Sargent M, York A, et al. ASK1 regulates cardiomyocyte death but not hypertrophy in transgenic mice. Circ Res 2009;105:1110-7. [Crossref] [PubMed]
- Shanmugam P, Valente A, Prabhu S, et al. Angiotensin-II type receptor and NOX2 mediate TCF/LEF and CREB dependent WISP1 induction and cardiomyocyte hypertrophy. J Mol Cell Cardiol 2011;50:928-38. [Crossref] [PubMed]
- Grieve DJ, Byrne J, Siva A, et al. Involvement of the nicotinamide adenosine dinucleotide phosphate oxidase isoform Nox2 in cardiac contractile dysfunction occurring in response to pressure overload. J Am Coll Cardiol 2006;47:817-26. [Crossref] [PubMed]
- Kang BY, Mehta JL. Rosuvastatin attenuates Ang II--mediated cardiomyocyte hypertrophy via inhibition of LOX-1. J Cardiovasc Pharmacol Ther 2009;14:283-91. [Crossref] [PubMed]
- Kubin AM, Skoumal R, Tavi P, et al. Role of reactive oxygen species in the regulation of cardiac contractility. J Mol Cell Cardiol 2011;50:884-93. [Crossref] [PubMed]
- Heusch P, Canton M, Aker S, et al. The contribution of reactive oxygen species and p38 mitogen-activated protein kinase to myofilament oxidation and progression of heart failure in rabbits. Br J Pharmacol 2010;160:1408-16. [Crossref] [PubMed]
- Schenkel PC, Tavares AM, Fernandes RO, et al. Redox-sensitive prosurvival and proapoptotic protein expression in the myocardial remodeling post-infarction in rats. Mol Cell Biochem 2010;341:1-8. [Crossref] [PubMed]
- Xuan YT, Tang XL, Banerjee S, et al. Nuclear factor- kappaB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res 1999;84:1095-109. [Crossref] [PubMed]
- Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol 2004;555:589-606. [Crossref] [PubMed]
- Engberding N, Spiekermann S, Schaefer A, et al. Allopurinol attenuates left ventricular remodeling and dysfunction after experimental myocardial infarction: a new action for an old drug? Circulation 2004;110:2175-9. [Crossref] [PubMed]
- Krijnen PA, Meischl C, Hack C, et al. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J Clin Pathol 2003;56:194-9. [Crossref] [PubMed]
- Looi YH, Grieve DJ, Siva A, et al. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension 2008;51:319-25. [Crossref] [PubMed]
- Zhao W, Zhao D, Yan R, et al. Cardiac oxidative stress and remodeling following infarction: role of NADPH oxidase. Cardiovasc Pathol 2009;18:156-66. [Crossref] [PubMed]
- Doerries C, Grote K, Hilfiker-Kleiner D, et al. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res 2007;100:894-903. [Crossref] [PubMed]
- Infanger DW, Cao X, Butler S, et al. Silencing Nox4 in the paraventricular nucleus improves myocardial infarction-induced cardiac dysfunction by attenuating sympathoexcitation and periinfarct apoptosis. Circ Res 2010;106:1763-74. [Crossref] [PubMed]
- Zhang Y, Sano M, Shinmura K, et al. 4-hydroxy-2-nonenal protects against cardiac ischemia-reperfusion injury via the Nrf2-dependent pathway. J Mol Cell Cardiol 2010;49:576-86. [Crossref] [PubMed]
- Borchi E, Parri M, Papucci L, et al. Role of NADPH oxidase in H9c2 cardiac muscle cells exposed to simulated ischaemia-reperfusion. J Cell Mol Med 2009;13:2724-35. [Crossref] [PubMed]
- Sucher R, Gehwolf P, Kaier T, et al. Intracellular signaling pathways control mitochondrial events associated with the development of ischemia/ reperfusion-associated damage. Transpl Int 2009;22:922-30. [Crossref] [PubMed]
- Sengupta A, Molkentin JD, Paik JH, et al. FoxO transcription factors promote cardiomyocytes survival upon induction of oxidative stress. J Biol Chem 2011;286:7468-78. [Crossref] [PubMed]
- Koga K, Kenessey A, Powell S, et al. Macrophage Migration Inhibitory Factor Provides Cardioprotection during Ischemia/Reperfusion by Reducing Oxidative Stress. Antioxid Redox Signal 2011;14:1191-202. [Crossref] [PubMed]
- Luo X, Cai H, Ni J, et al. c-Jun DNAzymes inhibit myocardial inflammation, ROS generation, infarct size, and improve cardiac function after ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol 2009;29:1836-42. [Crossref] [PubMed]
- Venardos KM, Zatta AJ, Marshall T, et al. Reduced L-arginine transport contributes to the pathogenesis of myocardial ischemia-reperfusion injury. J Cell Biochem 2009;108:156-68. [Crossref] [PubMed]
- Okazaki T, Otani H, Shimazu T, et al. Reversal of inducible nitric oxide synthase uncoupling unmasks tolerance to ischemia/reperfusion injury in the diabetic rat heart. J Mol Cell Cardiol 2011;50:534-44. [Crossref] [PubMed]
- Sandin A, Dagnell M, Gonon A, et al. Hypoxia followed by reoxygenation induces oxidation of tyrosine phosphatases. Cell Signal 2011;23:820-6. [Crossref] [PubMed]
- Dhalla AK, Hill MF, Singal PK. Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol 1996;28:506-14. [Crossref] [PubMed]
- Terentyev D, Gyorke I, Belevych AE, et al. Redox Modification of Ryanodine Receptors Contributes to Sarcoplasmic Reticulum Ca2+ Leak in Chronic Heart Failure. Circ Res 2008;103:1466-72. [Crossref] [PubMed]
- Dai DF, Johnson S, Villarin J, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res 2011;108:837-46. [Crossref] [PubMed]
- Borchi E, Bargelli V, Stillitano F, et al. Enhanced ROS production by NADPH oxidase is correlated to changes in antioxidant enzyme activity in human heart failure. Biochim Biophys Acta 2010;1802:331-8.
- Nediani C, Raimondi L, Borchi E, et al. Nitric oxide/reactive oxygen species generation and nitroso/redox imbalance in heart failure: from molecular mechanisms to therapeutic implications. Antioxid Redox Signal 2011;14:289-331. [Crossref] [PubMed]
- Li J, Solus J, Chen Q, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm 2010;7:438-44. [Crossref] [PubMed]
- Kim YM, Kattach H, Ratnatunga C, et al. Association of atrial nicotinamide adenine dinucleotide phosphate oxidase activity with the development of atrial fibrillation after cardiac surgery. J Am Coll Cardiol 2008;51:68-74. [Crossref] [PubMed]
- Kim YM, Guzik TJ, Zhang YH, et al. A myocardial Nox2 containing NAD(P)H oxidase contributes to oxidative stress in human atrial fibrillation. Circ Res 2005;97:629-36. [Crossref] [PubMed]
- Yagi S, Akaike M, Aihara K, et al. Endothelial nitric oxide synthase-independent protective action of statin against angiotensin II-induced atrial remodeling via reduced oxidant injury. Hypertension 2010;55:918-23. [Crossref] [PubMed]
- Chang JP, Chen MC, Liu WH, et al. Atrial myocardial nox2 containing NADPH oxidase activity contribution to oxidative stress in mitral regurgitation: potential mechanism for atrial remodeling. Cardiovasc Pathol 2011;20:99-106. [Crossref] [PubMed]
- Dudley SC Jr, Hoch NE, McCann LA, et al. Atrial fibrillation increases production of superoxide by the left atrium and left atrial appendage: role of the NADPH and xanthine oxidases. Circulation 2005;112:1266-73. [Crossref] [PubMed]
- Han W, Fu S, Wei N, et al. Nitric oxide overproduction derived from inducible nitric oxide synthase increases cardiomyocyte apoptosis in human atrial fibrillation. Int J Cardiol 2008;130:165-73. [Crossref] [PubMed]
- Bukowska A, Röcken C, Erxleben M, et al. Atrial expressionof endothelial nitric oxide synthase in patients with and without atrial fibrillation. Cardiovasc Pathol 2010;19:e51-60. [Crossref] [PubMed]
- Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013;34:3035-87. [Crossref] [PubMed]
- Maack C, Kartes T, Kilter H, et al. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Circulation 2003;108:1567-74. [Crossref] [PubMed]
- Shang F, Zhao L, Zheng Q, et al. Simvastatin inhibits lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal rat cardiomyocytes: The role of reactive oxygen species. Biochem Biophys Res Commun 2006;351:947-52. [Crossref] [PubMed]
- Thuc LC, Teshima Y, Takahashi N, et al. Mitochondrial K(ATP) channels-derived reactive oxygen species activate pro-survival pathway in pravastatin-induced cardioprotection. Apoptosis 2010;15:669-78. [Crossref] [PubMed]
- Obata T, Yonemoti H, Aomine M. The protective effect of fluvastatin on hydroxyl radical generation by inhibiting low-density lipoprotein (LDL) oxidation in the rat myocardium. Microvasc Res 2009;77:163-5. [Crossref] [PubMed]
- Di Napoli P, Taccardi A, Grilli A, et al. Chronic treatment with rosuvastatin modulates nitric oxide synthase expression and reduces ischemia-reperfusion injury in rat hearts. Cardiovasc Res 2005;66:462-71. [Crossref] [PubMed]
- Dobrev D, Nattel S. New antiarrhythmic drugs for treatment of atrial fibrillation. Lancet 2010;375:1212-23. [Crossref] [PubMed]
- Kovacs K, Hanto K, Bognar Z, et al. Prevalent role of Akt and ERK activation in cardioprotective effects of Ca(2+) channel- and beta-adrenergic receptor blockers. Mol Cell Biochem 2009;321:155-64. [Crossref] [PubMed]
- Lu JC, Cui W, Zhang H, et al. Additive beneficial effects of amplodipine and atorvastatin in reversing advanced cardiac hypertrophy in elderly spontaneously hypertensive rats. Clin Exp Pharmacol Physiol 2009;36:1110-9. [Crossref] [PubMed]
- Umemoto S, Tanaka M, Kawahara S, et al. Calcium anragonist reduces oxidative stress by upregulating Cu/Zn superoxide dismutase in stroke-prone spontaneously hypertensive rats. Hypertens Res 2004;27:877-85. [Crossref] [PubMed]
- Hwang JT, Kwon D, Park O, et al. Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutr 2008;2:323-6. [Crossref] [PubMed]
- Sebai H, Sani M, Aouani E, et al. Cardioprotective effect of resveratrol on lipopolysaccharide-induced oxidative stress in rat. Drug Chem Toxicol 2011;34:146-50. [Crossref] [PubMed]
- Shao ZH, Wojcik K, Dossumbekova A, et al. Grape seed proanthocyanidins protect cardiomyocytes from ischemia and reperfusion injury via Akt-NOS signaling. J Cell Biochem 2009;107:697-705. [Crossref] [PubMed]
- Li J, Liu H, Ramachandran S, et al. Grape seed proanthocyanidins ameliorate Doxorubicin-induced cardiotoxicity. Am J Chin Med 2010;38:569-84. [Crossref] [PubMed]
- Rajesh M, Mukhopadhyay P, Batkai S, et al. Xanthine oxidase inhibitor allopurinol attenuates the development of diabetic cardiomyopathy. J Cell Mol Med 2009;13:2330-41. [Crossref] [PubMed]
- Antoniades C, Shirodaria C, Van Assche T, et al. GCH1 haplotype determines plasma and vascular biopterin availability in coronary artery disease effects on vascular superoxide production and endothelial function. J Am Coll Cardiol 2008;52:158-65. [Crossref] [PubMed]
- Masano T, Kawashima S, Toh R, et al. Beneficial effects of exogenous tetrahydrobiopterin on left ventricular remodeling after myocardial infarction in rats: the possible role of oxidative stress caused by uncoupled endothelial nitric oxide synthase. Circ J 2008;72:1512-9. [Crossref] [PubMed]
- Silberman GA, Fan T, Liu H, et al. Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction. Circulation 2010;121:519-28. [Crossref] [PubMed]
- Antoniades C, Channon KM, Stefanadis C. Letter by Antoniades et al regarding article, "Uncoupled cardiac nitric oxide synthase mediates diastolic dysfunction Circulation 2010;122:e558. [Crossref] [PubMed]
- Moens AL, Champion H, Clayes M, et al. High-dose folic acid pretreatment blunts cardiac dysfunction during ischemia coupled to maintenance of high-energy phosphates and reduces postperfusion injury. Circulation 2008;117:1810-9. [Crossref] [PubMed]
- Bubb KJ, Kok C, Tang O, et al. The NRF2 activator DH404 attenuates adverse ventricular remodeling post-myocardial infarction by modifying redox signalling. Free Radic Biol Med 2017;108:585-94. [Crossref] [PubMed]
- Aonuma T, Takehara N, Maruyama K, et al. Apoptosis-Resistant Cardiac Progenitor Cells Modified With Apurinic/Apyrimidinic Endonuclease/Redox Factor 1 Gene Overexpression Regulate Cardiac Repair After Myocardial Infarction. Stem Cells Transl Med 2016;5:1067-78. [Crossref] [PubMed]


