Ground glass opacities management in the lung cancer screening era
Pulmonary ground glass opacity (GGO) is becoming an important clinical dilemma in oncology as its diagnosis in clinical practice is increasing. Moreover, the fact that low-dose computed tomography (CT) screening for lung cancer is being implemented in the United States and China and is under consideration in many other countries (1-3) will certainly increase in the future the identification of GGO. The trend has been confirmed in recent trials which reported a significant reduction of lung cancer mortality and overall mortality when lung screening is performed using low-dose CT (2,3). The incidence of cancer in GGO has been reported as high as 63%.
Heterogeneous biologic behavior can be present in different types of GGOs (4), which could also appear as a manifestation of focal interstitial fibrosis, aspergillosis, eosinophilic pneumonia, bronchiolitis obliterans and organizing pneumonia, endometriosis, Wegener granulomatosis.
Surgical criteria for GGOs are not well defined therefore, surgeons and oncologists often treat GGOs according to their own experience.
The purpose of this manuscript is to review the best available evidence papers on management of GGO in lung cancer to address the following questions: (I) how to correlate CT findings with malignancy; (II) which patient to operate on and when? (III) how to perform intraoperative detection of intrapulmonary GGO? (IV) results of surgical treatment.
Methods
Taking a cue from a clinical scenario a review on PubMed was conducted. The words searched included: ‘Lung ground glass opacity”. The research was limited to human and adults. We considered all published articles from 1990 to April 2017, which reported on at least sufficient data, to be eligible. The literature search was limited to articles in English. We evaluated the reports’ quality from the titles and abstracts. Exclusion criteria: no attempt was made to locate unpublished material. Reviews and teaching articles which contributed no data for analysis were also excluded, as they were case reports and small series or articles on pediatric patients.
Clinical scenario and results
One of our patients was operated 3 years ago for adenocarcinoma of the left upper lobe T2aN0M0. After a follow-up of 1 year, a GGO with some solid component was found in the remaining left lobe. The maximum standard uptake value (SUVmax) at CT/PET was low. We decided to offer a biopsy or wedge lung resection, however, the patient refused both procedures. Although after 2 years of its appearance the GGO is stable as shown in Figure 1, we remain in the opinion of biopsying or removing this GGO in the suspect malignancy.
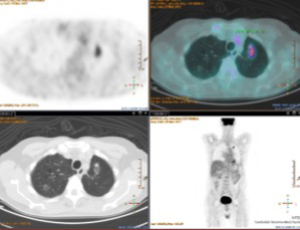
Looking through the literature, we have found 1,211 articles. Interestingly, while in 1991 only one paper was published on low-dose high-resolution CT (5), 126 papers have been published in 2016 clearly demonstrating a growing interest in GGO. Most cited and recent papers have been selected for discussion. Of note, the most recent papers have been published from Asian groups.
Definition
Pulmonary GGO is defined as an opacity that does not obscure the underlying structures or vessels at high resolution CT scan.
Classification
There is no conclusive classification of GGO. Some authors distinguish pure type and mixed type (6), other classify lung GGO in pure, heterogeneous and part-solid (7). The pure GGO has no central scar formation; the heterogeneous type is consolidated on lung window while the part-solid type is seen on mediastinal window.
How to correlate CT findings with malignancy?
In the real world, without biopsy, it is impossible to confirm if a lung nodule is benign or malignant, and the diagnosis of GGO is even more difficult because of its structure. For clinicians and surgeons, the obvious open question is: when is a GGO lung cancer? At this moment there is no rule, and it is very difficult to simplify as there could always be a interobserver variability, and in case of GGOs there is a particular difficulty to measure reproducibility (8).
In general, persistent findings of GGO on CT may be suggestive of a neoplastic condition with a higher probability of malignancy when a solid component is present. An enlarging solid lesion within a GGO could be an indicator of malignancy, while a lesion which disappears after antibiotics or steroids has a high probability to be a benign lesion. Moreover, lesions which remain stable during follow-up were often diagnosed histologically as focal interstitial fibrosis, atypical adenomatous hyperplasia (9). On the contrary, some authors suggest to suspect localized bronchioloalveolar carcinoma (BAC) and adenocarcinoma. Hasegawa et al. (10) demonstrated that the mean volume double times was 813, 457 and 149 days respectively in pure GGO, in lesions with central solid component or a solid appearance. The presence of GGO in a patient with adenocarcinoma may be due to the presence of a BAC component within the mass, and this was confirmed by Lee et al. in 100% of cases (11).
The precise value of PET/CT with HRCT to predict malignancy in a GGO is unclear. In the most cited meta-analysis, PET scan has relatively good sensitivity and specificity (96.8% and 77.8%, respectively) for distinguishing cancer from non-cancer in solid pulmonary nodules but the sensitivity of PET scan in subsolid nodules is lower (50%). However, it has been reported that BAC, which typically manifests as GGO on CT, is falsely negative on PET (12). Some studies have shown that the use of PET/CT to discriminate between benign and malignant GGO nodules is inappropriate, especially in the case of pure GGO nodules. The SUVmax of most pure GGO and some of mixed GGO is usually very low. A recent study by Zhou et al. (13) was performed retrospectively in 58 patients to examine PET/high-resolution CT for differentiating minimally invasive adenocarcinoma in stage IA lung cancer patients with solitary GGO. The study showed that GGO in the pre-invasive or minimally invasive adenocarcinoma group preferentially manifested as GGO (P<0.01) compared to the invasive adenocarcinoma group. The sensitivity in predicting invasive adenocarcinoma was higher with a combination of consolidation/tumor ratio >0.38 and a SUVmax >1.46 in mixed ground-glass opacity nodule when compared to those with SUVmax >0.95 alone or consolidation/tumor ratio >0.39 alone (both P>0.05). For a mixed GGO combined consolidation/tumor ratio >0.38 and SUVmax >1.46 appears to better predict invasive adenocarcinoma in stage IA lung cancer patients with solitary GGO.
Biopsy and detection of GGO
Preoperative biopsy
A “central” GGO of the lung represents a challenge for surgeons and radiologists. Although most surgeons recommend biopsy before resection, others prefer direct surgical resection. They support their practice on the basis of the high correlation between CT finding and histopathological finding and also the simplicity and the low invasiveness of VATS in removing a GGO. Nonetheless, it seems appropriate to have histology before surgery to organize the best treatment for the patient.
Shimizu et al. (14) evaluated, in a retrospective study, the value of preoperative percutaneous CT-guided fine needle aspiration biopsy (CTNB) in 96 patients with peripheral lung cancers smaller than 2 cm, especially in cases showing GGO aspect. The overall diagnostic yield of CTNB was 64.6%: 48.5% for lesions smaller than 10 mm, 62.5% for those 11–15 mm, and 83.9% for those of 16–20 mm, respectively. The diagnostic yields in GGO-dominant lesions (GGO ratio >50%) and solid-dominant lesions (GGO ratio <50%) were 51.2% and 75.6% (P=0.018). In the GGO-dominant group, the diagnostic yields were 35.2% for lesions smaller than 10 mm, 50.0% for those 11–15 mm, and 80.0% for those 16–20 mm. In the solid-dominant group, diagnostic yield was 62.5% for cases smaller than 10 mm, 75% for 11–15 mm and 85.7% for 16–20 mm, respectively. The authors concluded that CTBN is a useful diagnostic tool for peripheral small lung cancers; however, for GGO-dominant lesions, the preoperative diagnostic yield is not significantly better than for solid-dominant lesions.
Ikezawa et al. (15) have recently shown that endobronchial ultrasonography images and virtual bronchoscopy could be obtained for 156 (92%) of 169 GGO predominant-type lesions, and 116 (69%) were successfully diagnosed by this method [20 of 31 pure GGO lesions (65%); 96 of 138 mixed GGO predominant-type lesions (70%)]. The mean size of diagnosed lesions was significantly larger than that of non-diagnosed lesions (22 versus 18 mm, P<0.01). Regarding diagnostic yield based on CT, cases with presence of a bronchus leading directly to a lesion had significantly higher diagnostic yield than the other lesions (P<0.01).
Preoperative localization
Pure GGO are very difficult to be localized even on manual palpation, it is therefore evident that the finger localization during VATS of a central GGO is almost impossible. Localization is even more difficult when the lesion is deeper because the interference of hilar structures. Failure to palpate the nodule increased to 63% when the nodule was smaller than 10 mm and deep in the lung parenchyma. It is particularly difficult to locate semisolid or ground-glass opacity nodules surgically because they are difficult to palpate—even at thoracotomy. Suzuki et al. (16) reported 54% failure to visualize or palpate the nodule during VATS, and thoracotomy was necessary.
Although several localization techniques of GGO, with the aim to guide VATS resection, have been evaluated (methylene blue dye and placement of a hook wire); there is no consensus and there is no perfect technique to identify intraoperatively a GGO. Every surgeon chooses the method of biopsy and the VATS technique (17-21) more suitable for him. The known techniques are: (I) imaging modalities (ultrasonography and CT fluoroscopy); (II) injection of dyes, contrast media, radionuclides, or colored adhesive agents; (III) hook-wire placement. Some authors recently introduced a novel technique using a mixture of lipiodol and India ink for pleural tattooing for intraoperative localization of GGO patients who undergo a 4-cm working incision uniportal VATS. The authors injected the mixture of dye solution to the nearest area rather than on the target lesion to indicate the main pathologic lesion without contaminating it. Recently, some authors aimed to combine radiologically guided microcoil localization with palpation by using an oval forceps and wedge resection during uniportal VATS (22).
Which patient to operate on and when?
As there are no established guidelines on GGO, surgical indications are often based on personal experience. Many surgeons believe that a GGO should be removed only if it increases in size. When cancer is strongly suspected at the multidisciplinary meeting, a wait-and-see CT surveillance should be terminated regardless the percentage of the solid component or duration of the GGO. Suzuki et al. (23) suggest that peripheral lung nodules with a large GGO component, which do not disappear during follow-up, have a propensity to be carcinoma at the initial stage. In these patients, a less invasive resection via VATS should be performed. However, small changes in size or solid component of a nodule can be hardly recognized.
Different is the situation when GGO arises after resection for lung cancer as it could be a recurrence (Figure 1). Sawada et al. (24) showed that a higher consolidation relative to the tumor diameter (CTR) and an increase in CTR during follow-up were associated with invasive cancer. A follow-up period of 3 years is considered to be adequate for judging tumor growth in 16% of the patients with a CTR of 0. Aggressive cancer occurred in 4% of patients with a CTR of 0 and in 70% of patients with a CTR >25%. Aggressive cancer was observed in 46% of the patients whose CTR increased during the follow-up period and in 8% of the patients whose tumors increased in size. Cho et al. (25) have demonstrated that history of lung cancer, initial size of 8 mm or larger, presence of a solid component, and air bronchogram were independent risk factors for subsequent GGO growth, and therefore a longer follow-up period is necessary.
Wedge, segmentectomy or lobectomy for GGO?
Although lobectomy is the operation of choice to treat lung cancer, many surgeons perform limited resection such as segmentectomy or wedge lung resection to treat GGOs. Is this attitude correct? It is hard to say, but studies have demonstrated that limited resection for GGOs smaller than 20 mm have similar results to those obtained with standard lobectomy (26,27) while lobectomy is indicated for GGOs with over 25% of the solid component. Lee et al. (11) showed that a significant increase in size (over 2 mm) or the appearance of a solid portion may be an indication for major resection. A recent meta-analysis (28) demonstrated the indolent nature of GGO, and that lung cancers manifesting as GGO generally do not metastasize to lymph nodes or other organs. Nevertheless, the study has different weak points summarized as follows: (I) all the studies included in the meta-analysis were retrospective; (II) 22 out of 24 were studies from Asia; (III) there is no uniformity between studies in the decision making. There are also no data on multifocal GGO which could appear in the same lobe, lung or bilaterally.
Surgical strategy is certainly different when the surgeon prefers to go directly to resection (29). This attitude could be justified because, after the introduction of VATS, it could theoretically easier to remove a GGO. Nevertheless, a segmentectomy for a central GGO could be justified if the solid component is less than 2 cm.
Another point of discussion is the value of lymphadenectomy in GGO. Moon et al. (29) performed a retrospective study on 358 patients to analyze the effectiveness of mediastinal lymph node evaluation in patients with GGO tumor. The authors showed no difference in the 5-year recurrence-free survival rate among three groups in the GGO-predominant, and they concluded that MLE is not an essential procedure for clinical N0 NSCLC presenting as a 3 cm or smaller GGO-predominant nodule. Another interesting retrospective study was performed in 867 patients with GGO (28). All the lesions were classified into three groups according to the proportion of solid densities: group I, pure GGO; group II, 1% to 50%; group III, 50% to 79%. Twenty-five patients developed lymph node metastasis, among these 25 cases, 11 (11/160) were group II and 14 (14/154) were group III. The authors concluded that larger size, mixed GGOs with a higher proportion of solid component, and elevated serum CEA level were associated with a higher preference for nodal metastasis.
Guidelines
Recent guidelines have been published by different societies such as those of Fleischner Society (30) (Table 1), British Thoracic Society (31) or the Japan Clinical Oncology Group (JCOG) (32). According to the proposal of the JCOG strategy for small lung cancers with GGO characteristics, lobectomy should be performed for GGO larger than 3 cm, segmentectomy for lesions between 2 and 3 cm, and wedge resection for GGO smaller than 2 cm. Although a GGO less than 2 cm with a C/T ratio of 0.5–1.0 should undergo segmentectomy or wedge resection. Several prospective randomized trial studies are ongoing in Japan with the intention to find a definitive answer (JCOG0804, JCOG1211, JCOG0802).
Conclusions
Although data on GGOs are increasing, it is not possible to conclude what is the best strategy for GGO in the lung cancer screening era. While we wait the results of prospective randomized trials, personal opinion and experience of single physician should not drive decision making (33,34), on the contrary, at this moment “personalized” treatment should be decided by multidisciplinary oncology team.
Key points
- The increase of diagnose of GGO is creating a positive discussion on this new latecomer issue in oncology.
- Classification of GGO is not uniform, and therefore a global effort should be taken to “speak the same language”.
- A recent meta-analysis shows that the 5-year lung cancer specific survival rate is 100%. The data suggest a possible indolent course for lung cancer manifesting as GGO.
- A GGO which appears after surgery for lung cancer should be considered with different eyes as it could be nil, recurrence or a new cancer.
- Lymphadenectomy should be performed in larger size, mixed GGOs with a higher proportion of solid component.
- Single and multiple GGOs could have a different behavior.
- We propose to distinguish primary GGO (pGGO, when GGO appears as first instance) from secondary GGO (sGGO, when it appears during follow-up after resection for cancer).
- A global effort should be taken to uniform guidelines.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pedersen JH, Saghir Z, Wille MM, et al. Ground-Glass Opacity Lung Nodules in the Era of Lung Cancer CT Screening: Radiology, Pathology, and Clinical Management. Oncology (Williston Park) 2016;30:266-74. [PubMed]
- Silva M, Pastorino U, Sverzellati N. Lung cancer screening with low-dose CT in Europe: strength and weakness of diverse independent screening trials. Clin Radiol 2017;72:389-400. [Crossref] [PubMed]
- Ten Haaf K, Tammemägi MC, Bondy SJ, et al. Performance and Cost-Effectiveness of Computed Tomography Lung Cancer Screening Scenarios in a Population-Based Setting: A Microsimulation Modeling Analysis in Ontario, Canada. PLoS Med 2017;14:e1002225. [Crossref] [PubMed]
- Kobayashi Y, Mitsudomi T, Sakao Y, et al. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann Oncol 2015;26:156-61. [Crossref] [PubMed]
- Zwirewich CV, Mayo JR, Müller NL. Low-dose high-resolution CT of lung parenchyma. Radiology 1991;180:413-7. [Crossref] [PubMed]
- Nakata M, Saeki H, Takata I, et al. Focal ground-glass opacity detected by low-dose helical CT. Chest 2002;121:1464-7. [Crossref] [PubMed]
- Park CM, Goo JM, Lee HJ, et al. Nodular ground-glass opacity at thin-section CT: histologic correlation and evaluation of change at follow-up. Radiographics 2007;27:391-408. [Crossref] [PubMed]
- Detterbeck FC. More Opaque Than Clear: Reality Is Always Cloaked in Shades of Gray. Oncology (Williston Park) 2016;30:275-6. [PubMed]
- Lee CT. What do we know about ground-glass opacity nodules in the lung? Transl Lung Cancer Res 2015;4:656-9. [PubMed]
- Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol 2000;73:1252-9. [Crossref] [PubMed]
- Lee C, Guichet PL, Abtin F. Percutaneous Lung Biopsy in the Molecular Profiling Era: A Survey of Current Practices. J Thorac Imaging 2017;32:63-7. [Crossref] [PubMed]
- Gould MK, Maclean CC, Kuschner WG, et al. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA 2001;285:914-24. [Crossref] [PubMed]
- Zhou J, Li Y, Zhang Y, et al. Solitary ground-glass opacity nodules of stage IA pulmonary adenocarcinoma: combination of 18F-FDG PET/CT and high-resolution computed tomography features to predict invasive adenocarcinoma. Oncotarget 2017;8:23312-21. [PubMed]
- Shimizu K, Ikeda N, Tsuboi M, et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground-glass opacity at CT. Lung Cancer 2006;51:173-9. [Crossref] [PubMed]
- Ikezawa Y, Shinagawa N, Sukoh N, et al. Usefulness of Endobronchial Ultrasonography With a Guide Sheath and Virtual Bronchoscopic Navigation for Ground-Glass Opacity Lesions. Ann Thorac Surg 2017;103:470-5. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Migliore M, Calvo D, Criscione A, et al. Uniportal Video Assisted Thoracic Surgery: summary of experience, mini-review and perspectives. J Thorac Dis 2015;7:e378. [PubMed]
- Migliore M. Video-assisted thoracic surgery techniques for lung cancer: which is better? Future Oncol 2016;12:1-4. [Crossref] [PubMed]
- Migliore M. VATS surgery for anatomical lung resection: a different approach for every surgeon. Video-assist Thorac Surg 2016;1:31. [Crossref]
- Hansen HJ, Petersen RH. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - The Copenhagen experience. Ann Cardiothorac Surg 2012;1:70-6. [PubMed]
- Gonzalez-Rivas D. Uniportal thoracoscopic surgery: from medical thoracoscopy to non-intubated uniportal video-assisted major pulmonary resections. Ann Cardiothorac Surg 2016;5:85-91. [Crossref] [PubMed]
- Shi Z, Chen C, Jiang S, et al. Uniportal video-assisted thoracic surgery resection of small ground-glass opacities (GGOs) localized with CT-guided placement of microcoils and palpation. J Thorac Dis 2016;8:1837-40. [Crossref] [PubMed]
- Suzuki K, Asamura H, Kusumoto M, et al. "Early" peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg 2002;74:1635-9. [Crossref] [PubMed]
- Sawada S, Yamashita N, Sugimoto R, et al. Long-term Outcomes of Patients With Ground-Glass Opacities Detected Using CT Scanning. Chest 2017;151:308-15. [Crossref] [PubMed]
- Cho J, Ko SJ, Kim SJ, et al. Surgical resection of nodular ground-glass opacities without percutaneous needle aspiration or biopsy. BMC Cancer 2014;14:838. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Rami-Porta R, Tsuboi M. Sublobar resection for lung cancer. Eur Respir J 2009;33:426-35. [Crossref] [PubMed]
- Zha J, Xie D, Xie H, et al. Recognition of "aggressive" behavior in "indolent" ground glass opacity and mixed density lesions. J Thorac Dis 2016;8:1460-8. [Crossref] [PubMed]
- Moon Y, Sung SW, Namkoong M, et al. The effectiveness of mediastinal lymph node evaluation in a patient with ground glass opacity tumor. J Thorac Dis 2016;8:2617-25. [Crossref] [PubMed]
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Baldwin DR, Callister ME. Guideline Development Group. The British Thoracic Society guidelines on the investigation and management of pulmonary nodules. Thorax 2015;70:794-8. [Crossref] [PubMed]
- Aokage K, Saji H, Suzuki K, et al. A non-randomized confirmatory trial of segmentectomy for clinical T1N0 lung cancer with dominant ground glass opacity based on thin-section computed tomography (JCOG1211). Gen Thorac Cardiovasc Surg 2017;65:267-72. [Crossref] [PubMed]
- Treasure T, Utley M, Hunt I. When professional opinion is not enough. BMJ 2007;334:831-2. [Crossref] [PubMed]
- When the evidence is weak, caution should be applied. Lancet Oncol 2010;11:805. [Crossref] [PubMed]



