Nrf2: a promising trove for diabetic wound healing
An extensively re-establishing and complex process of cutaneous wound healing arises to maintain the disrupted skin barrier function which encompasses interdependent stages like hemostasis, inflammation, proliferation and tissue remodelling (1). This well-orchestrated, multistep process of wound healing is impeded in diabetic patients resulting into chronic wounds, creating several therapeutic complications which pose severe health burden to the global population. Peripheral neuropathy, vascular insufficiency, shortage of oxygenation, bacterial infections, increased oxidative stress, elevation in matrix metalloproteinases activity and diminished levels of growth factors are the array of contributing factors in interrupted and delayed diabetic wound healing (2). Substantial evidences have established a plausible relationship between elevated oxidative stress and retarded wound healing in type I and type II diabetic patients (3,4). Reactive oxygen species (ROS) under physiological range are beneficial to attenuate the invasion of microbes and also to regulate some intracellular signalling pathways (5); but when their levels are raised drastically, process of wound healing gets trapped in an unregulated and self-sustaining phase of inflammation which also contribute to protein, lipid and DNA damage resulting in escalated cell damage.
Nuclear related factor 2 (Nrf2), also called nuclear related (erythroid-derived 2) like 2 (NFE2L2) is widely studied and well known nuclear transcription factor. It is a basic leucine zipper (bZIP) protein and its primary function is to provide defense against cellular oxidative stress by increasing the transcription of various endogenous antioxidants by binding to antioxidant response element (ARE) of genome. This protein is sequestered by a cytosolic repressor factor: Kelch-like ECH associated protein 1 (Keap1) which targets it for proteasomal degradation. As depicted in Figure 1, when oxidative stress shoots up, Nrf2 leaves Keap1 and enters into the nucleus mitigating the effects of oxidative stress by increasing the expression of endogenous antioxidant defense enzymes such as HO-1, NQO1 (6). Recent literature evidences have clearly shown the protective effect of 4-ethyl catechol and 4-vinyl catechol against diabetic wound healing via augmentation of Nrf2 mediated antioxidant defenses (7).
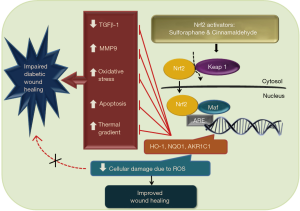
Min Long and colleagues have reported that Nrf2 plays a crucial role to elevate the levels of endogenous antioxidants like heme oxygenase 1 (HO-1), aldo-keto reductase family 1 member C1 (AKR1C1) and NAD(P)H quinone dehydrogenase 1 (NQO1) and hastens the rate of diabetic wound healing (8). In this study, authors have tested the effect of diet derived nontoxic Nrf2 activators: Sulforaphane (SF) and Cinnamaldehyde (CA) on the rate of diabetic wound healing (8). Negative role of magnified oxidative stress in perilesional skin tissue of diabetic patients has already been discussed in literature. Here, authors reported that there is amplified apoptosis, DNA damage due to oxidative stress and transient increase in compensatory Nrf2 and HO-1, NQO1 genes in diabetic patients in initial stages of wound healing. This rise in Nrf2 activity does not last longer in diabetic patients and creates necessity for the compensation of impaired antioxidant defense mechanism. In in vivo studies, rate of wound healing in STZ induced diabetic Nrf2−/− C57BL/6 mice was slower than in Nrf2+/+ mice because deprivation of Nrf2 leads to inadequacy in antioxidant defense response. Treatment with 12.5 mg/kg SF and 50 mg/kg CA resulted in augmented Nrf2 activation which further resulted into faster reduction in wound diameter via Nrf2 directed elevated expression levels of HO-1, AKR1C1, NQO1. Importantly, Nrf2 expression was not significantly altered in normoglycemic lesioned mice indicating SF and CA exert their activity of inducing expression and activation of Nrf2 in diabetic mice only.
Along with alteration in Nrf2 levels, increased transforming growth factor-β1 (TGF-β1) expression has been reported in SF and CA treated mice. TGF-β1 signaling has a crucial role in new skin layer formation and it is involved in various processes like re-epithelialization, inflammation, angiogenesis and overall epidermal maintenance (9). As studied by Gökşen and co-authors, application of recombinant human PDGF-BB (platelet derived growth factor-BB) around wound area has shown to increase the antioxidant levels in early phase of diabetic wound healing of rats (10). Also, moderate expression of MMP9 and decline in DNA damage due to 8-oxo-dG (8-Oxo-2’-deoxyguanosine) were observed in treatment group compared to diabetic control, indicating the anti-inflammatory, tissue protective mechanisms of SF and CA activity (8). MMP9 is the enzyme belonging to matrix metalloproteinase (MMP9) family. During an inflammatory phase of wound healing, it removes damaged extracellular matrix and allows tissue contraction and remodelling. It is essential in certain amounts; but elevated levels of MMP9 can allow excessive matrix degradation, resulting into impaired wound healing (11). Thus, moderate levels of MMP9 are required for typical process of wound healing.Apart from this, TUNEL assay showed more apoptosis in STZ treated mice but that was drastically reduced in SF/CA treated animals.
In in vitro study, on giving treatment of 20 µmol/L CA to human immortalized keratinocytes (HaCaT cells) in high glucose medium, accelerated wound closure in wound healing assay was observed. This pharmacological activation of NRF2 promotes keratinocyte proliferation and migration but inhibits apoptosis. Also, there was increase in antioxidant defense which consequently mitigated apoptosis and regulated the levels of MMP9.
Collectively, looking at all results, we can say Nrf2 is not only responsible for alleviating tissue damage due to oxidative stress but also it regulates re-epithelialization and can positively modulate levels of essential proteins involved in wound healing process.
According to WHO, global prevalence of diabetes had reached up to 8.5% in 2014. Fifteen percent to twenty-five percent of this population is susceptible to develop foot ulcers (12). Hence, there is a compelling necessity to develop novel therapeutic strategies to mitigate the development of diabetes associated foot ulcers. Increasing the expression of TGF-ß, reducing apoptosis, giving phototherapy, use of bioengineered skin substitutes, stem cell therapy, formulations of vascular endothelial growth factor (VEGF) are some of the various available approaches to alleviate diabetic wounds. These approaches are insufficient to control the development of ever increasing incidences of diabetic wounds and it remains as unmet clinical need. Therefore, the work done by Min Long and colleagues can offer some novel aspects to the existing literature and supports the therapeutic usefulness of Nrf2 activators in diabetic wound healing. Since, Nrf2 is involved in multiple facets of physiological processes associated with wound healing and its reduction has also been observed to cause peripheral neuropathy (13): a prequel to foot ulcers, targeting Nrf2 may offer novel and better alternative for the therapeutic management of wounds in diabetic patients. Current evidence can be taken as a basis for the beneficial effects of Nrf2 mediated tissue protection and should be further evaluated for developing futuristic strategies for diabetic wound management.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736-43. [Crossref] [PubMed]
- Devalliere J, Dooley K, Hu Y, et al. Co-delivery of a growth factor and a tissue-protective molecule using elastin biopolymers accelerates wound healing in diabetic mice. Biomaterials 2017;141:149-60. [Crossref] [PubMed]
- Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes 1991;40:405-12. [Crossref] [PubMed]
- Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation. Glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and ageing. Biochem J 1988;256:205-12. [Crossref] [PubMed]
- Schäfer M, Werner S. Oxidative stress in normal and impaired wound repair. Pharmacol Res 2008;58:165-71. [Crossref] [PubMed]
- Kumar A, Mittal R. Nrf2: a potential therapeutic target for diabetic neuropathy. Inflammopharmacology 2017;25:393-402. [Crossref] [PubMed]
- Senger DR, Cao S. Diabetic Wound Healing and Activation of Nrf2 by Herbal Medicine. J Nat Sci 2016;2:e247. [PubMed]
- Long M, Rojo de la Vega M, Wen Q, et al. An Essential Role of NRF2 in Diabetic Wound Healing. Diabetes 2016;65:780-93. [Crossref] [PubMed]
- Ramirez H, Patel SB, Pastar I. The Role of TGFβ Signaling in Wound Epithelialization. Adv Wound Care (New Rochelle) 2014;3:482-91. [Crossref] [PubMed]
- Gökşen S, Balabanlı B, Coşkun-Cevher Ş. Application of platelet derived growth factor-BB and diabetic wound healing: the relationship with oxidative events. Free Radic Res 2017;51:498-505. [Crossref] [PubMed]
- Ayuk SM, Abrahamse H, Houreld NN. The Role of Matrix Metalloproteinases in Diabetic Wound Healing in relation to Photobiomodulation. J Diabetes Res 2016;2016:2897656. [Crossref] [PubMed]
- Tchero H, Herlin C, Bekara F, et al. Failure rates of artificial dermis products in treatment of diabetic foot ulcer: A systematic review and network meta-analysis. Wound Repair Regen 2017;25:691-6. [Crossref] [PubMed]
- Negi G, Kumar A, Sharma SS. Nrf2 and NF-κB modulation by sulforaphane counteracts multiple manifestations of diabetic neuropathy in rats and high glucose-induced changes. Curr Neurovasc Res 2011;8:294-304. [Crossref] [PubMed]


