Outcomes following transcatheter aortic valve replacement in patients with native aortic valve regurgitation
Introduction
Severe symptomatic native aortic valve regurgitation (NAVR) is associated with poor prognosis (1,2). Surgical aortic valve replacement (SAVR) is presently the treatment of choice according to current guidelines (3). However, for inoperable patients, very few treatment options are available. Recently, transcatheter aortic valve replacement (TAVR) has become a standard of care for inoperable and high-risk patients with severe symptomatic aortic stenosis (4-6). However, NAVR is still generally considered a relative contraindication for TAVR. This is due to the non-calcified aortic annulus in this population leading to the presumed risk of device failure from inadequate anchoring of the transcatheter valve prosthesis (7). Despite the relative contra-indication of TAVR in NAVR, there have been a few published reports (8-11) that continue to show that TAVR in carefully selected patients with NAVR is feasible with good outcomes especially in the high surgical risk population. This study is a systematic review analyzing current data on short and mid-term outcomes of TAVR in patients with NAVR.
Methods
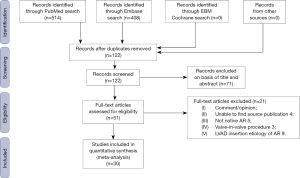
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews recommended by the Cochrane Collaboration was followed in this study (Figure 1). A systematic search of all relevant report published in English from 2002 to 2016 documenting outcomes following TAVR in NAVR, including case reports, case series, and original reports, was performed in the PubMed, Embase and Cochrane database of systematic reviews. A Boolean search was performed combining the following key words: “transcatheter aortic valve replacement” or “transcatheter aortic valve implantation” AND “aortic regurgitation”. No language restriction was applied. We scanned the bibliographies of all included articles and relevant review articles to identify additional studies. Only studies reporting data on demographic, procedural characteristics, management and follow up were included. To obtain missing data, the primary investigators of the included studies were contacted. All publications were limited to those involving human subjects. Conference presentations, ongoing studies, editorials, reviews and expert opinions were excluded. Statistical analysis was done using CMA Version 3.3.070 (Bio Stat Inc., Englewood, NJ, USA). Two authors (Siri Kadire and Tamunoinemi Bob-Manuel) screened and retrieved reports and excluded irrelevant studies. Any uncertainty about the eligibility of any included study was resolved by two other authors (MR Heckle and UN Ibebuogu) and group consensus. Aortic valve regurgitation was assessed by echocardiography using color flow doppler, vena contracta, pressure half-time, and defined as grade 0 (none), grade 1 (trace), grade 2 (mild), grade 3 (moderate), and grade 4 (severe) (12). The method of aortic regurgitation grading was not specified in all the included studies. Primary analysis of the data was performed by measuring frequencies and descriptive statistics, including mean and standard deviation. We compared the reported 30-day mortality of included studies with a forest plot. Heterogeneity among studies was assessed with I2 statistic. We also tested for publication bias by creating a funnel plot using the Begg and Egger methods (13,14).
Results
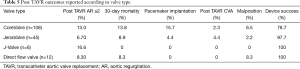
A total of 30 publications (Table 1) describing 182 patients were included in our study; 54% male, mean age 70.1±2.6 years (Table 2). The mean logistic European System for Cardiac Operative Risk Evaluation score (EuroSCORE) was 21.8%±4.5%. Aortic valve calcification was absent in 87% of the patients, while the rest had non-significant calcification. In our study population valve degeneration accounted for 68% of NAVR, while endocarditis, aortic aneurysm, and degeneration post valve repair accounted for 9%, 7%, 6%, respectively (Table 3). The self-expanding CoreValve device (Medtronic, Inc., Minneapolis, Minnesota, USA) was used in 59% of cases, JenaValve (JenaValve Technology, Munich, Germany) was used in 25% of patients and Direct flow valve (Direct Flow Medical, Santa Rosa, California, USA) was used in 6% of the patients. Other valve types altogether made up the remaining 10%. The reported TAVR access site was mainly femoral (58.8%) and transapical (33.1%), with carotid, subclavian and transaortic access making up the remaining 8%. The mean aortic annular diameter was 22.6±0.97 and 24.3±1.28 mm in patients whose valves were measured with echocardiography and multi-slice computed tomography, respectively (Table 2).
Full table

Full table

Full table
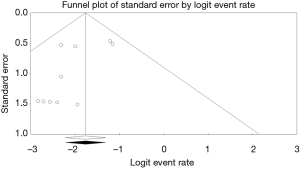
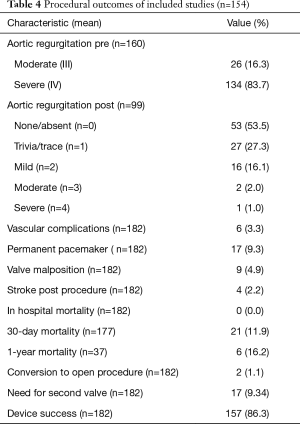
Clinical outcomes are summarized in Tables 4,5. Device success was achieved in 86.3% of our study population. Pacemaker implantation was required post-procedure in 9.3% of patients. The 30-day and 1-year mortality was 11.9% (n=177) and 16.2% (n=37), respectively. Grade 1 and less post-procedure aortic regurgitation occurred in 81%, while moderate to severe post procedural aortic regurgitation occurred in 3% of the study population. The permanent pacemaker implantation rate was 9.3% while post-procedural stroke occurred in 2.2%. The 30-day and 1-year mortality were 11.9% (n=177) and 16.2% (n=37), respectively. Some of the included studies did not report one-year mortality data. Conversion to open surgery occurred in 1.29% of the study population. Major vascular complications defined as aortic dissections, major hemorrhage and major structural complications occurred in 3.3% of cases. Despite 86.8% of the study population having NAVR without valve calcification, the mean device success was 86.3%. A funnel plot showed no apparent asymmetry for 30-day mortality outcomes (Figure 2). Publication bias was assessed with Egger’s score (t-value 0.00, P=0.758), and was not significant.
Full table
Full table
Discussion
Severe aortic regurgitation is associated with excess mortality and high morbidity. Within 10 years of diagnosis, 75% of the patients had died or required aortic valve replacement (1). The incidence of aortic regurgitation increases after the age of 50 years (15) and published data (7,11) suggests that the most frequent cause of aortic regurgitation is native valve degeneration as confirmed in our study. Also, congenital disorders like bicuspid aortic valve predispose patients to aortic valve degeneration (16). Aortic regurgitation can lead to several complications including progressive left ventricular dysfunction and dilation, congestive heart failure, myocardial ischemia, arrhythmia, and death. Surgery is currently considered the gold standard in the treatment for chronic severe aortic regurgitation in the presence of symptoms or impaired left ventricular function (3,17). However, for inoperable patients, the therapeutic options are limited. As shown in the Euro heart survey, advanced age and multiple comorbidities are common reasons for non-surgical management with a resultant annual mortality rate of 10% to 20% (18). The main challenge in implementing TAVR in this population is due to the absence of calcification in the device landing zone, which may lead to suboptimal fixation of the lower part of the valve prosthesis at the annulus during deployment, resulting in device malposition. Also, the increased movement of the native valve in the regurgitant jet and dilation of the aortic root can affect procedural success (11). Hence, NAVR has been considered a contraindication to TAVR (5). With the advent of newer valve designs that are fully retrievable and repositionable (19), and valves with feelers and clips (20), which can provide structural support for their placement even in the absence of calcium, there is optimism going forward. However, more research is needed to create a valve more specific to the challenges of NAVR.
In our analysis, 54% had no aortic regurgitation post procedure, compared to 3% who had moderate to severe post procedure AR (≥ grade 2). Significant residual aortic regurgitation can eventually result in poor clinical outcomes in the future especially due to the relatively younger age of this population. The published data by Roy et al. (11) and Testa et al. (9) showed high rates of valve-in-valve (19% and 30%) and residual aortic regurgitation (21% and 88%, respectively). This was likely related to insufficient over-sizing, inability to reposition the valve and insufficient anchoring.
In our study population, post-TAVR prosthetic regurgitation was reported less with the JenaValve (6.7%) (Table 5) (10,21-23). The JenaValve, which is made of a self-expandable nitinol stent appears to overcome many of the issues encountered by other valve types due to its unique clip fixation to the native leaflets and feeler guided positioning system that attaches to the native aortic valve leaflets, stabilizing implantation even in the absence of annular or leaflet calcifications (22). Over-sizing of the CoreValve may reduce post-procedural regurgitation, however this is still insufficient in eradicating it as shown in the reviewed reports. The majority (56%) of the included studies (9,11,24) in our analysis utilized the CoreValve self-expanding device because of the ability of self-expandable valves to offer high and permanent recoil forces, making it better suited for treating aortic regurgitation. However, the percentage of post-TAVR regurgitation is high showing that the first generation CoreValve may not be adequate for this function. Although, the oversizing of the CoreValve is necessary for valve anchoring especially in the absence of calcification, it is known to be a predictor of conduction abnormalities post procedure and need for pacemaker implantation (25-27). Also, oversizing carries the risk of annular rupture especially with aggressive post-dilatation, which is a rare event that can be fatal. In addition to Aortic stenosis (28), JenaValve has shown good preliminary results for aortic regurgitation as well (4,5). The JenaValve is now the only TAVR device approved for the treatment of high-risk or inoperable patients with severe AR. The Direct Flow Valve System (Direct Flow Medical, Santa Rosa, California, USA), which can be implanted via transfemoral, subclavian, or direct aortic access, has a unique anchoring mechanism with two inflatable ring balloons filled with a contrast saline mixture and a polymer that solidifies after inflation (19). The Direct Flow valve’s full functionality during positioning allows real time repositioning and reduces residual regurgitation noticed after placement. The stability of the Direct Flow valve can be ascertained after implantation using the positioning wires before performing the polymer exchange and permanently fixing the device. Like the CoreValve, adequate oversizing of the Direct Flow valve helps to secure it in place especially in the non-calcified annulus. Two of the studies included in our analysis (19,29), although with small sample sizes, used the Direct Flow valve with impressive outcomes with a relatively low 8.3% post-TAVR regurgitation, 100% device success and no post-TAVR stroke or pacemaker implantation was seen (Table 5). However, the Direct Flow valve is not commercially available presently.
The self-expandable Symetis ACURATE TA device (Symetis, Ecublens, Switzerland) features a self-positioning system, which offers tactile feedback and comes with the unique feature of self-positioning at a supra-annular level (20). Our review contained eight patients from one study (6) with good outcomes.
Other valves types such as the J-Valve system (JC Medical, US), Engager valve (Medtronic) (1%), and Sapien XT (Edwards Lifesciences, Irvine, California, USA) (1%) were rarely used in our reviewed reports. Although they all showed encouraging results with minimal post-TAVR regurgitation and acceptable 30-day mortality, it is difficult to draw conclusions from single case reports (30-32) or a singular patient in a case series (24). They however show the future possibilities of a wide array of TAVR devices that can be used and further developed in aortic regurgitation patients.
Certain factors may make patients with native aortic regurgitation particularly suitable for transcatheter therapies. In aortic stenosis, catheter manipulations of the calcified aortic valve and the diseased aorta seem to be associated with an increase in stroke risk (33). The absence of aortic leaflet calcifications in many cases of aortic regurgitation may therefore lower the risk for thromboembolic events during TAVR. However, the relatively high rate of stroke in this patient population may be related to other non-cardiac factors such as atrial fibrillation and cardiac septal defects. Secondly, balloon aortic valvuloplasty pre-TAVR or post dilatation of implanted prosthesis is rarely needed and may lower the risk for conduction disturbances related to the procedure compared to TAVR for calcified aortic valve stenosis (6). Contrary to the theoretical risk of instability of the TAVR in the absence of device landing zone calcifications in NAVR, the lack of calcification did not hinder device success, which was 86% in our analysis despite 87% of included patients having no valvular calcification.
Limitations of study
This is a retrospective analysis of 30 separate clinical studies; hence it retains the limitations of each individual study design. There was a wide variation in the number of patients enrolled in the individual studies, from 43 patients in Roy et al. (11) where mean outcomes were calculated and analyzed, to several case reports that described single patient outcomes. Six prosthetic valve types were assessed with five different access sites (transfemoral, apical, subclavian, carotid and transaortic), but outcomes were not always reported along these lines. This may be a confounding factor to patient outcomes, although we think short-term mortality would be similar among these groups. Included in this analysis were clinical studies from 2002 to September 2016. Hence only outcomes using 1st generation CoreValve (FDA approved Nov 2007) are included. There were no published studies using the second-generation CoreValve Evolut R or the Edwards Sapien 3 in high-risk patients to treat Aortic regurgitation. Finally, the inherent lack of randomization in the design of the included studies means that the current analysis is susceptible to selection bias.
Conclusions
TAVR in NAVR is associated with acceptable pacemaker implantation rate, in-hospital and 1-year mortality rate. However, there is room for improvement. Randomized control trials will be needed to validate the findings of our study. There is surprisingly good device success even with the lack of aortic calcifications in majority of included patients (84%) thought to be integral to prosthesis stabilization in NAVR. However larger studies are still needed to properly analyze and assess long-term structural and mortality outcomes associated with TAVR in this patient population before we can generalize results.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Dujardin KS, Enriquez-Sarano M, Schaff HV, et al. Mortality and morbidity of aortic regurgitation in clinical practice. A long-term follow-up study. Circulation 1999;99:1851-7. [Crossref] [PubMed]
- Tarasoutchi F, Grinberg M, Spina GS, et al. Ten-year clinical laboratory follow-up after application of a symptom-based therapeutic strategy to patients with severe chronic aortic regurgitation of predominant rheumatic etiology. J Am Coll Cardiol 2003;41:1316-24. [Crossref] [PubMed]
- Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012). The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). G Ital Cardiol (Rome) 2013;14:167-214. [PubMed]
- Mack MJ, Leon MB, Smith CR, et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet 2015;385:2477-84. [Crossref] [PubMed]
- Holmes DR Jr, Mack MJ, Kaul S, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement: developed in collaboration with the American Heart Association, American Society of Echocardiography, European Association for Cardio-Thoracic Surgery, Heart Failure Society of America, Mended Hearts, Society of Cardiovascular Anesthesiologists, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Ann Thorac Surg 2012;93:1340-95. [Crossref] [PubMed]
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N Engl J Med 2016;374:1609-20. [Crossref] [PubMed]
- Bekeredjian R, Grayburn PA. Valvular heart disease: aortic regurgitation. Circulation 2005;112:125-34. [Crossref] [PubMed]
- Wendt D, Kahlert P, Pasa S, et al. Transapical transcatheter aortic valve for severe aortic regurgitation: expanding the limits. JACC Cardiovasc Interv 2014;7:1159-67. [Crossref] [PubMed]
- Testa L, Latib A, Rossi ML, et al. CoreValve implantation for severe aortic regurgitation: a multicentre registry. EuroIntervention 2014;10:739-45. [Crossref] [PubMed]
- Seiffert M, Bader R, Kappert U, et al. Initial German experience with transapical implantation of a second-generation transcatheter heart valve for the treatment of aortic regurgitation. JACC Cardiovasc Interv 2014;7:1168-74. [Crossref] [PubMed]
- Roy DA, Schaefer U, Guetta V, et al. Transcatheter aortic valve implantation for pure severe native aortic valve regurgitation. J Am Coll Cardiol 2013;61:1577-84. [Crossref] [PubMed]
- Zoghbi WA, Enriquez-Sarano M, Foster E, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 2003;16:777-802. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Maurer G. Aortic regurgitation. Heart 2006;92:994-1000. [Crossref] [PubMed]
- Roberts WC, Vowels TJ, Ko JM. Natural history of adults with congenitally malformed aortic valves (unicuspid or bicuspid). Medicine 2012;91:287-308. [Crossref] [PubMed]
- Klodas E, Enriquez-Sarano M, Tajik AJ, et al. Optimizing timing of surgical correction in patients with severe aortic regurgitation: role of symptoms. J Am Coll Cardiol 1997;30:746-52. [Crossref] [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Schofer J, Nietlispach F, Bijuklic K, et al. Transfemoral Implantation of a Fully Repositionable and Retrievable Transcatheter Valve for Noncalcified Pure Aortic Regurgitation. JACC Cardiovasc Interv 2015;8:1842-9. [Crossref] [PubMed]
- Kempfert J, Rastan AJ, Mohr FW, et al. A new self-expanding transcatheter aortic valve for transapical implantation - first in man implantation of the JenaValve™. Eur J Cardiothorac Surg 2011;40:761-3. [PubMed]
- Zhu D, Chen Y, Zhang J, et al. Transapical implantation of a new second-generation transcatheter heart valve in patients with pure aortic regurgitation: a preliminary report. Interact Cardiovasc Thorac Surg 2015;20:860-2. [Crossref] [PubMed]
- Seiffert M, Diemert P, Koschyk D, et al. Transapical implantation of a second-generation transcatheter heart valve in patients with noncalcified aortic regurgitation. JACC Cardiovasc Interv 2013;6:590-7. [Crossref] [PubMed]
- Schlingloff F, Frerker C, Schäfer U, et al. Transapical aortic valve (JenaValve) implantation for severe aortic insufficiency and aortic aneurysm. J Thorac Cardiovasc Surg 2013;146:e40-1. [Crossref] [PubMed]
- Frerker C, Schewel J, Schewel D, et al. Expansion of the indication of transcatheter aortic valve implantation--feasibility and outcome in "off-label" patients compared with "on-label" patients. J Invasive Cardiol 2015;27:229-36. [PubMed]
- Bax JJ, Delgado V, Bapat V, et al. Open issues in transcatheter aortic valve implantation. Part 2: procedural issues and outcomes after transcatheter aortic valve implantation. Eur Heart J 2014;35:2639-54. [Crossref] [PubMed]
- Nazif TM, Dizon JM, Hahn RT, et al. Predictors and clinical outcomes of permanent pacemaker implantation after transcatheter aortic valve replacement: the PARTNER (Placement of AoRtic TraNscathetER Valves) trial and registry. JACC Cardiovasc Interv 2015;8:60-9. [Crossref] [PubMed]
- van der Boon RM, Nuis RJ, Van Mieghem NM, et al. New conduction abnormalities after TAVI--frequency and causes. Nat Rev Cardiol 2012;9:454-63. [Crossref] [PubMed]
- Treede H, Mohr FW, Baldus S, et al. Transapical transcatheter aortic valve implantation using the JenaValve™ system: acute and 30-day results of the multicentre CE-mark study. Eur J Cardiothorac Surg 2012;41:e131-8. [Crossref] [PubMed]
- Mangieri A, Latib A, Aurelio A, et al. Successful implantation of a second-generation aortic valve in severe aortic regurgitation secondary to a traumatic cusp lesion. Cardiovasc Revasc Med 2015;16:429-31. [Crossref] [PubMed]
- Wei L, Liu H, Zhu L, et al. A New Transcatheter Aortic Valve Replacement System for Predominant Aortic Regurgitation Implantation of the J-Valve and Early Outcome. JACC Cardiovasc Interv 2015;8:1831-41. [Crossref] [PubMed]
- Cerillo AG, Griese D, Berti S. Successful percutaneous implantation of symetis ACURATE neo transcatheter aortic bioprosthesis for the treatment of pure aortic regurgitation. Catheter Cardiovasc Interv 2016;88:319-23. [Crossref] [PubMed]
- Kiefer P, Seeburger J, Mohr FW, et al. Transcatheter aortic valve replacement for isolated aortic valve insufficiency: experience with the Engager valve. J Thorac Cardiovasc Surg 2014;147:e37-8. [Crossref] [PubMed]
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-98. [Crossref] [PubMed]