Semaglutide—the “new kid on the block” in the field of glucagon-like peptide-1 receptor agonists?
Introduction
It is a known fact that, due to the progressive nature of type 2 diabetes (T2D) and decline of beta cell function, diabetes patients require over time treatment intensification to maintain good glycemic control (1,2). In addition, international treatment guidelines recommend achieving glycemic targets while minimizing the risk of hypoglycemia and weight gain, two of the most frequent adverse effects of “classical” diabetes medications (1,2). The last decade witnessed the advent of the glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs), one of the most promising classes of “modern” diabetes medications (3). GLP-1RAs decrease glycaemia by stimulating insulin secretion from the pancreatic beta cells in a glucose dependent manner and inhibiting glucagon secretion from the pancreatic alpha cells, in the same time promoting satiety (direct effect on cerebral GLP-1 receptors and prolongation of gastric emptying), and, consequently, weight loss (4).
According to their mode of action, GLP-1RAs can be classified into short-acting preparations with once (lixisenatide) or twice (eventide) daily administration and long-acting with once daily (liraglutide) or once weekly (QW) administration (eventide QW, dulaglutide, albiglutide and semaglutide) (5). While long-acting GLP-1RAs have a stronger effect on fasting plasma glucose and overall glycemic control, short-acting preparations decrease more post-prandial blood-glucose (3,5). In addition, once-weekly GLP-1RAs may improve patient compliance and quality of life compared to the short-action preparations (6). Head-to-head clinical trials as well as systematic reviews/meta-analyses showed that long-acting GLP1RAs are superior to short-acting GLP-1RAs in reducing HbA1c (7). Moreover, the compounds with long duration of action proved to be superior to basal insulin in reducing HbA1c as shown by recent meta-analyses (5,8).
Semaglutide development
Semaglutide is a GLP-1RA in the final phases of authorization for the treatment of T2D in humans. Semaglutide has a high degree of homology (~94%) with the human GLP-1 molecule, rendering it a human GLP-1 analogue (9). There are three key changes in the molecule of semaglutide compared to human GLP-1: alanine in position 8 is replaced by α-aminoisobutyric acid (which increases resistance to DPP-4 action); a C-18 fatty acid is conjugated to Lys in position 26 via a glutamate spacer, providing specific binding to albumin; and Lys in position 34 is substituted by Arg (preventing the C-18 fatty acid to bind at a wrong site) (10). After s.c. injection, the half-life of semaglutide is approximately one week and an early phase 2 randomized study showed that doses to be used in clinical trials should be 0.5 and 1.0 mg (11). A formulation of semaglutide with oral administration is currently in the phase 3 clinical trials period (12).
Semaglutide efficacy and safety in clinical trials
Now, in The Lancet Diabetes Endocrinology, Sorli and colleagues report the results of SUSTAIN 1 (NCT02054897), a 30-week, phase 3, randomized controlled trial investigating the efficacy and safety of semaglutide monotherapy versus placebo in treatment naïve T2D patients uncontrolled by lifestyle changes alone (Table 1) (13). Overall 388 patients were randomized 2:2:1:1 to once-weekly semaglutide (0.5 or 1.0 mg) or injected placebo. Mean baseline age was 53.7 years, mean diabetes duration 4.18 years and mean HbA1c 8.05%. Patients were obese (mean BMI 32.93 kg/m2) with a good sex and race balance.
After 30 weeks of treatment, HbA1c decreased significantly with both semaglutide doses, the placebo subtracted difference being 1.43% (0.5 mg dose), respectively 1.53% (1.0 mg semaglutide). Overall 74% of patients treated with 0.5 mg semaglutide and 72% of those with 1.0 mg semaglutide reached a target HbA1c of <7%, while 59%, respectively 60% attained a HbA1c ≤6.5%. Most importantly, this target was achieved without severe or blood-glucose-confirmed hypoglycemia.
In the same time, treatment with semaglutide was associated with a significant weight loss: −3.73 kg for the 0.5 mg semaglutide treated patients and −4.53 kg for the 1.0 mg semaglutide group, in both cases significantly better compared to placebo treated patients.
In SUSTAIN-1, treatment with the dose of 1.0 mg of semaglutide was also associated with a significant decrease (compared to placebo) in total and LDL-cholesterol as well as in the levels of free fatty acids. In addition to the decrease with the 2–3 mmHg of the systolic blood pressure (SBP), this completes the favorable CV safety profile of semaglutide which was already proven in patients with already established cardiovascular disease (CVD) or very high cardiovascular (CV) risk in the SUSTAIN-6 trial (18).
Otherwise, treatment with semaglutide was rather well tolerated, with a profile of adverse events comparable with that already reported for GLP-1RAs (7), with gastrointestinal (GI) side effects (nausea and diarrhea especially) being most often reported. Overall prevalence of GI side effects was 38% for both doses of semaglutide compared with 15% in placebo treated subjects. There were no episodes of pancreatitis reported during the follow-up treatment.
Three other major randomized controlled studies with semaglutide already published their full results. These are SUSTAIN-2 in which efficacy and safety of semaglutide compared with sitagliptin was tested in T2D patients treated with metformin, a thiazolidinedione (TZD) or both (19), SUSTAIN-4 in which the efficacy and safety of semaglutide was compared with that of basal insulin glargine in T2D patients treated with metformin plus minus a sulphonylurea (SU) (20) and, finally, SUSTAIN-6 which tested de CV safety of semaglutide in patients with high CVD risk (18). Interestingly, the hypoglycemic effects of semaglutide were quite consistent across these studies, despite the fact they included rather different patient populations in various stages of T2D evolution. Thus, mean HbA1c decrease varied between 1.1% and 1.3% for the 0.5 mg dose and between 1.4% and 1.64% for the 1.0 mg dose (18-20), comparable with the 1.45%, respectively 1.55% recorded in SUSTAIN-1. The same is true for the percentage of subjects reaching a HbA1c <7%, 72–74% in the SUSTAIN-1 trial, between 60% (0.5 mg dose) and 78% (1.0 mg dose) in the other three semaglutide studies. Regarding the weight loss, this again was consistently reproducible, ranging between 3.5 and 4.3 kg for the 0.5 mg dose and between 5 and 6.1 kg for the 1.0 mg dose (18-20). Interestingly, in a post-hoc analysis of the DURATION-6 data (21) it was shown that weight loss is a direct effect of semaglutide and not related with the GI side effects (nausea and vomiting) associated with GLP1-RA treatment.
Concerning adverse events, the most frequently reported in all SUSTAIN trials were GI side effects, the prevalence ranging from 40% to 50%, partially dependent with the duration of exposure to semaglutide. However, usually these were mild in intensity and very rarely (3–5%) led to discontinuation of study medication (18-20).
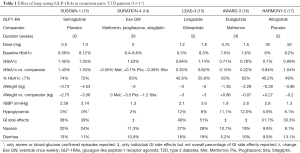
While differences in the study populations included in clinical trials and trial design per se make it difficult to compare the findings of different randomized controlled trials (RCTs), we are giving in Table 1 the main results of the studies that used other long acting GLP-1RAs in T2D patients that were treatment naïve at the time of randomization.
As shown, semaglutide led to similar HbA1c decreases compared with once weekly eventide (however higher baseline HbA1c for Exe QW) and higher than once daily liraglutide and once weekly albiglutide (both with similar HbA1c at baseline). Once weekly dulaglutide was also associated with a lower drop in HbA1c compared to semaglutide but lower HbA1c at baseline makes difficult to interpret this difference. Overall, semaglutide was associated with the highest decrease in weight but also with a higher incidence of nausea compared with the other once weekly GLP-1RAs (24% for 0.5 mg semaglutide, 11.3% for Exe QW, 19% for 1.5 mg dulaglutide and 9.1% for 50 mg albiglutide).
Obviously more relevant are the results of head to head clinical trials comparing these molecules. Thus, the SUSTAIN-3 trial (22) compared the efficacy and safety of once weekly (QW) semaglutide 1.0 mg with that of Exe QW on 813 T2D subjects not controlled on oral drugs (HbA1c at baseline 8.3%). After 56 weeks of treatment, semaglutide reduced HbA1c significantly greater than Exe QW: 1.5% compared with 0.9%, with 67% of semaglutide treated patients reaching the HbA1c target of <7% compared with 40%. In the same time, patients treated with semaglutide lost significantly more weight compared with those treated with Exe QW: mean body weight reduction 5.6 vs. 1.9 kg. However, semaglutide was associated with a higher percentage of GI adverse events (41.8% vs. 33.3%) while Exe QW had more injection site reactions (1.2% for semaglutide vs. 22% for Exe QW) (22).
More recently, a press release from Novo Nordisk (23) communicated some of the results of the SUSTAIN-7 trial which compared the efficacy and safety of semaglutide QW 0.5 and 1.0 mg with that of dulaglutide QW 0.75 and 1.5 mg. The trial included 1201 T2D subjects uncontrolled on metformin alone, with a mean HbA1c at baseline of 8.2% and a mean body weight of 95 kg. After 40-week of treatment, 0.5 mg of semaglutide QW reduced HbA1c by 1.5% compared to 1.1% for 0.75 mg of dulaglutide QW, while 1.0 mg of semaglutide QW reduced HbA1c by 1.8% vs. 1.4% for dulaglutide QW 1.5 mg. The HbA1c target of <7% was achieved by 79% of patients treated with 1.0 mg of semaglutide compared with 68% of the patients treated with 1.5 mg dulaglutide. Moreover, semaglutide was associated with significantly higher reduction of body weight: 4.6 vs. 2.3 kg for 0.5 mg semaglutide and 0.75 mg of dulaglutide, 6.5 vs. 3 kg for 1.0 mg semaglutide, respectively 1.5 mg dulaglutide (23).
Conclusions
Overall, the results of the SUSTAIN trial program, including SUSTAIN-1, indicate a robust efficacy of once weekly semaglutide in improving metabolic control and decreasing body weight, with a safety profile characteristic for a GLP-1RA, in two trials semaglutide QW being superior to other two GLP-1RAs with once weekly administration: exenatide and dulaglutide. This combines with the results of the SUSTAIN-6 trial indicating the CV benefits of semaglutide: significant 26% reduction (P<0.001) of the composite of CV death, non-fatal myocardial infarction and non-fatal stroke for T2D patients treated with semaglutide versus placebo (18).
So, currently, five GLP-1RAs are already approved for the treatment of T2D while semaglutide is in the final phases of approval. Some significant differences exist between the existing GLP-1RAs and semaglutide seems to have some advantages over currently existing drugs. However, finally, the choice of a specific GLP-1RA will be based on several factors, including patient preferences, potential adverse effects, and cost.
Acknowledgements
None.
Footnote
Conflicts of Interest: C Guja has received consulting fees from Alfa Wasserman, AstraZeneca, Bayer AG, Berlin-Chemie Mennarini, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Sanofi. R Dănciulescu Miulescu has no conflicts of interest to declare.
References
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140-9. [Crossref] [PubMed]
- American Diabetes Association. Pharmacologic approaches to glycemic treatment. sec. 8. in standards of medical care in Diabetes-2017. Diabetes Care 2017;40:S64-S74. [PubMed]
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies. Ther Adv Endocrinol Metab 2015;6:19-28. [Crossref] [PubMed]
- ten Kulve JS, Veltman DJ, van Bloemendaal L, et al. Endogenous GLP-1 mediates postprandial reductions in activation in central reward and satiety areas in patients with type 2 diabetes. Diabetologia 2015;58:2688-98. [Crossref] [PubMed]
- Abd El Aziz MS, Kahle M, Meier JJ, et al. A meta-analysis comparing clinical effects of short- or long-acting GLP-1 receptor agonists versus insulin treatment from head-to-head studies in type 2 diabetic patients. Diabetes Obes Metab 2017;19:216-27. [Crossref] [PubMed]
- Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab 2017;19:1242-51. [Crossref] [PubMed]
- Htike ZZ, Zaccardi F, Papamargaritis D, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in type 2 diabetes: A systematic review and mixed-treatment comparison analysis. Diabetes Obes Metab 2017;19:524-36. [Crossref] [PubMed]
- Singh S, Wright EE Jr, Kwan AY, et al. Glucagon-like peptide-1 receptor agonists compared with basal insulins for the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Obes Metab 2017;19:228-38. [Crossref] [PubMed]
- Lau J, Bloch P, Schäffer L, et al. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J Med Chem 2015;58:7370-80. [Crossref] [PubMed]
- Lorenz M, Evers A, Wagner M. Recent progress and future options in the development of GLP-1 receptor agonists for the treatment of diabesity. Bioorg Med Chem Lett 2013;23:4011-8. [Crossref] [PubMed]
- Nauck MA, Petrie JR, Sesti G, et al. A phase 2, randomized, dose-finding study of the novel once-weekly human GLP-1 analog, semaglutide, compared with placebo and open-label liraglutide in patients with type 2 diabetes. Diabetes Care 2016;39:231-41. [PubMed]
- Tran KL, Park YI, Pandya S, et al. Overview of glucagon-like peptide-1 receptor agonists for the treatment of patients with type 2 diabetes. Am Health Drug Benefits 2017;10:178-88. [PubMed]
- Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once-weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double-blind, randomised, placebo-controlled, parallel-group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol 2017;5:251-60. [Crossref] [PubMed]
- Russell-Jones D, Cuddihy RM, Hanefeld M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care 2012;35:252-8. [Crossref] [PubMed]
- Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009;373:473-81. [Crossref] [PubMed]
- Umpierrez G, Tofé Povedano S, Pérez Manghi F, et al. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care 2014;37:2168-76. [Crossref] [PubMed]
- Nauck MA, Stewart MW, Perkins C, et al. Efficacy and safety of once-weekly GLP-1 receptor agonist albiglutide (HARMONY 2): 52 week primary endpoint results from a randomised, placebo-controlled trial in patients with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetologia 2016;59:266-74. [Crossref] [PubMed]
- Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med 2016;375:1834-44. [Crossref] [PubMed]
- Ahrén B, Masmiquel L, Kumar H, et al. Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as an add-on to metformin, thiazolidinediones, or both, in patients with type 2 diabetes (SUSTAIN 2): a 56-week, double-blind, phase 3a, randomised trial. Lancet Diabetes Endocrinol 2017;5:341-54. [Crossref] [PubMed]
- Aroda VR, Bain SC, Cariou B, et al. Efficacy and safety of once-weekly semaglutide versus once-daily insulin glargine as add-on to metformin (with or without sulfonylureas) in insulin-naive patients with type 2 diabetes (SUSTAIN 4): a randomised, open-label, parallel-group, multicentre, multinational, phase 3a trial. Lancet Diabetes Endocrinol 2017;5:355-66. [Crossref] [PubMed]
- Consoli A, Bain SC, Davies M, et al. Semaglutide provides sustained reductions in body weight over 2 years in subjects with type 2 diabetes (SUSTAIN 6). Diabetologia 2017;60:S4.
- Ahmann A, Capehorn M, Charpentier G, et al. Efficacy and safety of once-weekly semaglutide vs. exenatide ER in subjects with type 2 diabetes (SUSTAIN 3). Diabetes 2016;65:A49.
- Novo Nordisk A/S. Semaglutide superior to dulaglutide on glucose control and weight loss in people with type 2 diabetes in SUSTAIN 7. Novo Nordisk A/S 2017.Available online: https://globenewswire.com/news-release/2017/08/16/1086742/0/en/Semaglutide-superior-to-dulaglutide-on-glucose-control-and-weight-loss-in-people-with-type-2-diabetes-in-SUSTAIN-7.html