Quickly quantifying the dissolution fingerprints of compound Danshen dropping pill by HPLC
Introduction
The right way of quality control of Herbal drug (HD) and Traditional Chinese Medicine (TCM) will be make use of all their constituents quantified simultaneously by chromatographic fingerprints, for which our research group have been finishing a series of theories and techniques and also computer software for scientific evaluation (1-3). The investigatement of all component dissolution is one of the most important characteristics for a HD or TCM, for which directly affects the drug absorption and bioavailability (4,5). According to the related instruction principle, most of TCM needn’t do the dissolution test in its quality control but detect the disintegration time (6-11), for which may make TCM quality control blind and nonscientific in some extents. Compound Danshen Dropping Pills (CDDP) is composed of Radix Salviae Miltiorrhiae, Radix Notoginseng and Broneolum Syntheticum (12), which is the most important modern TCM with a nearly 4 billion yuans production per year. Today, CDDP is most widely used as an OTC medicine for treating, preventing before the cardiovascular and cerebrovascular disease and also for the emergency medical treatment (13-16). Since 1997, CDDP came into Food and Drug Administration (FDA) and enter the II, III stage of IND as the first TCM. The sales of CDDPs accounted more than 3 billion yuans per year in nearly ten years and ranked the first in TCM market (17-20). It is very needed further studying the new quality control method from the important indicatrix of CDDP to reflect the dissolution process for providing the previous information of its absorption in vivo, though there was chromatographic fingerprint to qualitatively examine CDDP consistency in fingerprint distribution (21) that have not been the best adequate quality control method.
The paper presented here expounded on employing the SQFM to quantitatively identify both quality and the dissolution process changes in vitro tests, in order to ensure CDDP stability and reproducibility in quality, in which the HPLC-FPs and DFPs in vitro were simultaneously established to possibly comprehensively analyze HDs or TCMs as the most ideal quality control method.
Applying the SQFM to assess CDDP-FPs (22,23)
It was a very effective way to control the TCM by quantified fingerprint technology because of TCM being the complicated science characteristics. In order to control the whole quality for CDDPs, SQFM performed the analyses of CDDPs from the macro qualitative similarity(MQLS) Sm that could give a full view of quantity and distribution ratio for TCM-CFP, seen
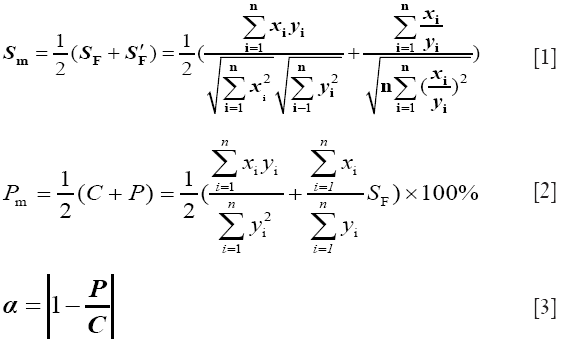

Full Table
Experimental section
Instruments
The analysis was performed on an Agilent 1100 series LC system (Hewlett Packard, CA, USA) consisting of a diode array detector, a low-pressure quaternary pumps, an online degasser and an autosampler. All data acquired were preceded by ChemStation workstation (Agilent technology Inc.). KQ-50B ultrasonic bath (Kunshan ultrasonic instrument company limited), RE52 rotary evaporator (Yarong biochemistry instrument factory, Shanghai, China) was used during the analysis.
Chemicals
Methanol (purchased from Yuwang industry limited company, Shandong, China) and glacial acetic acid (provided by Kemiou chemicals developing center, Tianjin, China) were both of HPLC grade. Water used for buffer solution was deionized and other chemicals were of analytical grade. PHA, DSS and SAB were all purchased from National Institute for the Pharmaceutical and Biological Products (Beijing, China, for quantified grade). CDDPs wrere from Tianjin Tasly Pharmaceutical Co.,Ltd (Tianjin, China).
Sample and standard preparation
Sample solutions
10 pills were accurately weighed and extracted with 50 mL 50% (V/V) ethanol for 1 hour. Then the filtrate was concentrated to approximately 10 mL under reduced pressure in a rotary evaporator. Finally, 50% (V/V) methanol was added to make the volume of 25 mL.
Standard solutions
PHA of 5.0 mg was accurately weighed and 50% (V/V) methanol was added to make 25 mL exactly to serve as the standard solution after shaking. DSS and SAB solutions were prepared in the same way.
Chromatographic condition
Chromatographic separation was carried out on a CenturySIL C18 BDS column (250 mm × 4.6 mm, 5 µm). The mobile phase consisted of 1% acetic acid in water (A) and methanol with 1% acetic acid (B) using a gradient program of 10-32% B in 0-15 mins; 32-45% B in 15-30 mins; 45-80% B in 30-50 mins. The flow rate was 1.0 mL/min. Detection wavelength was set at 290 nm to record the chromatograms. The column temperature was kept at (30±0.15) °C. An aliquot of 10 µL sample solution was loaded for analyses.
Results and discussion
Method suitability tests
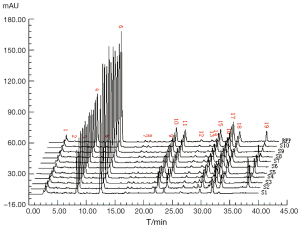
The DSS, PHA, SAB standard solutions and sample solution were injected for 10 µL respectively and recorded the chromatograms. By comparing the retention times of peaks and ultraviolet spectrograms in the sample and standard chromatograms, we drew the conclusion that the peak of 8.64 mins was from DSS (peak 4), 12.90 mins was from PHA (peak 6) and the peak of 37.52 mins was from SAB (peak 19). The above chromatograms were shown in Figure 1. For the good separation with the neighboring peaks and its adequate retention time, the peak of PHA was selected as the reference peak.
Injection precision test
The sample solution was injected six times successively and recorded the chromatograms. The RSDs of the relative retention times of all fingerprint peaks were below 1.0% and the RSDs of relative peak areas were all below 3.0% when PHA was selected as the reference, which indicated the method with a good injection precision.
Stability of the solution
The sample solution was injected after preparing for 0, 2, 4, 8 and 12 hrs, and recorded the chromatograms respectively. The RSDs of the relative retention times of all fingerprint peaks were less than 2.0%, and the relative peak areas were less than 3.0%, in which PHA was selected as the reference, indicating that the sample solution was stable within 12 hours.
Method reproducibility
Six replicates of CDDP were prepared according to the procedure described in section “Sample and Standard Preparation” to inject and record the chromatograms. The RSDs of the relative retention time of all peaks were below 1.0%, and that of the relative peak area was within 3.0%, which showed that the method reproducibility was satisfactory.
The establishment and evaluation of CDDP-FPs
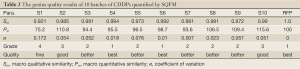
The sample solutions of 10 batches of CDDPs were prepared according to the procedure described above, loaded into the HPLC system and recorded the chromatograms, seen Figure 1. By comparing the ten CDDP-FPs, we marked 19 co-possessing peaks, in which the appearance ratio of each common peak was only needed more than 70%. The RFP was set up in terms of calculating the mean of the 10 batch of CDDP fingerprint signals, for which severed as the norm to evaluate their quality results listed in Table 2. In regard to the 10 batches of CDDPs, the qualities of S4 and S6 were belong to best (grade 1), those of S3, S5, S7, S8 and S9 belong to better (grade 2), those of S2 and S10 belong to good (grade 3), and only S1 falling to fine (grade 4) with lower contents of all 19 peak components and a larger variance coefficient. Hence the complicated TCM sample were comprehensively briefly quantified to give the authentic evaluation results, in which SQFM was demonstrated to be the best way for identifying herbal drugs and TCM quality.
Full Table
The development of DFPs
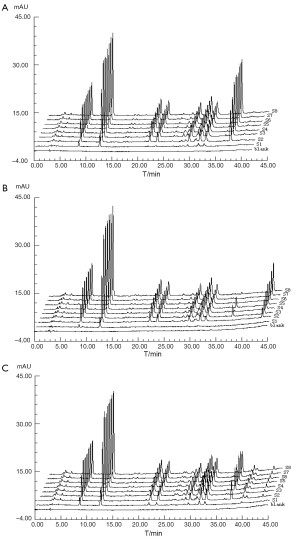
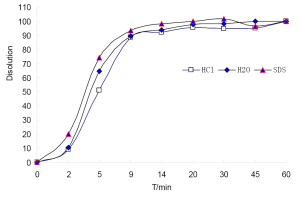
In dissolution test, a Chp cup method was used at a rotational speed of 100 rpm and at a temperature of 37.0±0.5 °C. Dissolution media (100 mL) were respectively the water, 0.1 mol/L hydrogen chloride (HCl) and 0.5% sodium dodecyl sulphate (SDS) which were de-gassed. Accurately weighed 20 pills of CDDP were subsequently added to each of the dissolution cups. At 2, 5, 9, 14, 20, 30, 45, and 60 min, samples were pipetted and replaced with 2 mL volume of dissolution media. The solution was immediately filtered through 0.45 µm Millipore filter and then determined by HPLC to record the chromatograms. Being low concentration of sample, the results showed that there were 1, 1, 2 peaks were missing in 0.1 mol/L hydrogen chloride (HCl), 0.5% sodium dodecyl sulphate (SDS) and water, respectively, seen Figure 2.
In this study, Sm and Pm were chosen to evaluate the DFPs and judge the dissolution stability both from the entirety and the mainly important indicatrix. All the chromatograms integrated of CDDP in three different dissolution media were loaded into the 4.0 edition software:the digitized evaluation system of the supper information characteristcs for TCM-FPs invented by Guoxiang SUN, etc., in which we took the chromatogram at final time point for the referential fingerprint dissolved thorougly. The fingerprint quantity evaluation results were seen in Figure 3 to display that CDDP was dispersed very slowly in 0.1 mol/L HCl, perhaps happening to degradation of some components from the Sm in 9 and 20 mins points. Comparing the sum of peak area of three different dissolution media, water finished the quick dissolution rate, 0.5% SDS solution was secondary and that was the last in 0.1 mol/L HCl according to the Pm of the three different dissolution media, plotted in Figure 4.
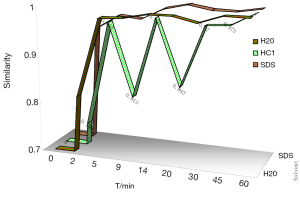
Fitting rule of Pm in CDDP dissolution process
The integrated releasing behavior of CDDP could further be investigated by constructing the fitting equations. According to Weibull function, dissolution equations were fitted by lnln [1/(1–Pm)] via lnt to draw the results as fellows, in water: lnln [1/(1–Pm)]=–0.5951x2+3.8146x–4.5195, r=0.9974; In 0.1 mol/L HCl solution: lnln [1/(1–Pm)]=–0.5555x2+3.5814x–4.6126, r=0.9972; and the fitting equation in 0.5% SDS solution was not shown, because there were up and down changes leading to a discordant tendency. Finally, water was chosen as the optimal dissolution media for its well dissolution stability and high dissolution efficiency, in which we could get the accumulation dissolution rate of CDDP at any time point according to the fitting equation. The dissolution parameters were followed: T50=4.02 mins (released 50%), Td=4.80 mins (released 63.2%), T90=7.968 mins (released 90%), so the overall ability of CDDP in water should dissolved more than 50% in 4.1 minutes and more than 90% in 8.0 minutes.
Investigating the variation of the four strong dissolution fingerprint peaks of CDDPs
The therapeutic outcome of TCM was due to the coherent effects of chemical constituents at equal pace (9,10). All the components certainly took the same important roles in TCM therapeutic process, so the dissolution rule of important chemical constituents was studied separately in order to guide the preparation technology and improve their bioavaibility. We investigated the four strong peaks of CDDP-DFP and constructed corresponding fitting equation. The relative stripping curves of the four strong peaks were shown in Figure 3 and fitting equations of four strong peaks were as follow, if let lnln [1/(1–Ai/Amax)]=y, the first strong peak (No.6 peak, PHA for 12.9 mins), y=–0.5603x2+3.4516x–3.8974, r=0.9988; the second strong peak (No.19 peak, SAB for 37.5 mins), y=–1.7127x2+9.6655x–11.947, r=0.9897; the third strong peak (No.4 peak, DSS for 8.6 mins), y=–0.4239x2+3.0436x–3.6276, r=0.9985; the forth strong peak (No.17 peak, unkown), y=–0.5019x2+3.3706x–4.1053, r=0.9979. The fitting equations of the four strong peaks were gained for specially monitoring the dissolution dynamic changes of the 4 important fingerprint peaks that had the same dissolution tendency on whole except SAB the second strong peak.
Conclusions
Comparing with the western medicine, compound recipe of TCM for medication not only had its own special feature, but also made chemical component complicated and the indicatrix composition uncertain that limited the study and research of quality control for TCM. In this paper, basing on the CDDP-HPLC-FPs, we separately studied the vitro dynamic process of CDDP and the four strong peaks of DFP in water for finding a better way to wholly control CDDP quality and monitor the dissolution state for each fingerprint component. The DFP can better reflect the holo-dissolution characteristics through the fitted equation, for which served as novel method to measure the dissolution dynamic process to make sure the concentration of CDDP in vitro or in vivo under control all time. Furthermore, we constructed four fitting equations for the four strong peaks and observed what difference existed in their dissolution process. DFPs could provide how stable and fast were for the most important fingerprints and supply much important information about their dissolution rule. In a word, we put forth a good way to evaluate the HD or TCM quality both from the wholly quantitative analyses and the quantitative DFPs that can post the best adequate way for controlling the quality of HD or TCM.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Sun GX, Hou ZF, Zhang CL, et al. Comparison between the qualitative similarity and the quantitative similarity of chromatographic fingerprints of traditional Chinese medicines. Yao Xue Xue Bao 2007;42:75-80. [PubMed]
- Sun GX, Shi XF, Zhang JX, et al. Determining the fingerprint attribution ratio and process recovery of medicinal effectiveness components for TCM-compound by quantified fingerprint method. Yao Xue Xue Bao 2008;43:1047-52. [PubMed]
- Sun GX, Hu YX, Bi KS. Evaluating the quality of niuhuangjiedu tablets by the systematic quantified fingerprint method. Yao Xue Xue Bao 2009;44:401-5. [PubMed]
- Liu JY, Lee KF, Sze CW, et al. Intestinal absorption and bioavailability of traditional Chinese medicines: a review of recent experimental progress and implication for quality control. J Pharm Pharmacol 2013;65:621-33. [PubMed]
- Zhang J, Huang Y, Liu D, et al. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur J Pharm Sci 2013;48:740-7. [PubMed]
- Jin JP. The research and application of dissolution test in drug quality control. Anhui Med Pharm 2006;09:699-702.
- Lv CH. The present situation and prospect of drug dissolution test method in China. Anhui Med Pharm 2006;09:537-8.
- Jiang Y, Tian SX. The dissolution review of Chinese Traditional medicine. Chin Tradit Pat Med 2006;28:415-9.
- Zhao M. Research review for dissolution of traditional Chinese medicine in solid state. Anhui Med Pharm 2007;11:171-3.
- Kale K, Hapgood K, Stewart P. Drug agglomeration and dissolution--what is the influence of powder mixing? Eur J Pharm Biopharm 2009;72:156-64. [PubMed]
- Pabla D, Akhlaghi F, Zia H. A comparative pH-dissolution profile study of selected commercial levothyroxine products using inductively coupled plasma mass spectrometry. Eur J Pharm Biopharm 2009;72:105-10. [PubMed]
- Ch. P. 2010;I:906.
- Jiang GR, Chen WM. Clinical investigation on compound Danshen dropping pill in treatment of coronary heart disease and angina pectoris. Chin Mod Med 2010;10:53-4.
- Li XF, Liu F, Jiang TL, et al. Efficacy of compound Danshen drop pill in 42 patients with early acute myocardial infarction. Chin New Drug J 2010;18:1699-702.
- Li YG, Song L, Liu M, et al. Advancement in analysis of Salviae miltiorrhizae Radixet Rhizoma (Danshen). J Chroma A 2009;1216:1941-53.
- Kang DG, Oh H, Chung HT, et al. Inhibition of angiotensin converting enzyme by lithospermic acid B isolated from Radix Salviae miltiorrhiza Bunge. Phytother Res 2003;17:917-20. [PubMed]
- Ding N. Research progress and clinical application of Compound Danshen Dripping Pills. Chin Tradit Herb Drugs 2002;33:1147.
- Yan XJ. Reflection and Practice for modernization of traditional Chinese medicine. Chin Tradit Pat Med 2000;22:863-4.
- Guo ZX. Modernization of Compound Danshen Dropping Pills. Chin J Infor Tradt Chin Med 2000;7:14-5.
- Wu Y. Compound Danshen Dropping Pills successfully enter the II stage of IND in America. J Tradit Chin Med 2010;51:817.
- Sun GX, Song YQ. Quality evaluation of compound Danshen dropping pills by systematic quantified fingerprint method based on HPLC fingerprints. Zhongnan Yaoxue 2009;7:297-300.
- Sun GX, Ren PP, Bi YM. Quality Assessment on High Performance Liquid Chromatographic Fingerprints of Ginkgo Leaf Extract and Dipyridamole Injection by Double Qualitative Similarities and Double Quantitative Similarities. Chin J Chromatogr 2007;25:518-23.
- Sun G, Zhi X, Zhang C, et al. Digitized evaluation system for super-information characteristics of chromatographic fingerprint in traditional Chinese medicine. Zhongnan Yaoxue 2007;5:550-5.