Antiangiogenic therapy and immune checkpoint blockade go hand in hand
Immune checkpoint blockade (ICB) therapies, such as the ones targeting cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) or programmed cell death protein 1 (PD-1), have displayed durable clinical responses in various cancers and are now approved by the FDA for a growing list of cancer types (1). Although these new immunotherapies have had a notable effect on cancer treatment, multiple mechanisms of immune resistance have been described. Tumors considered immunologically “cold”, with reduced or even absent infiltration of T-cells, are a major hurdle in the cancer immunotherapy field, and strategies to overcome this resistance mechanism constitute an unmet need. Recent efforts have focused on combining this immunotherapy approach with other therapies to maximize its potential. However, the candidate for these combination therapies are numerous and therefore the need for mechanism based and rationally designed therapies is needed.
In this manuscript by Allen et al., they propose to combine ICB with antiangiogenic therapy. The interest in targeting tumor vasculature has been pursued by multiple groups and the role of blood vessels in tumor progression has been investigated for more than a century (2). The requirement of a blood supply in order to sustain tumor development and its capacity to metastasize is a critical feature of cancer (3). However, tumor vasculature is generally known to be unevenly distributed and chaotic, leading to blood flow through tumors that do not follow a constant, unidirectional path. This creates zones of ischemia and ultimately necrosis as tumors outgrow their blood supply (4). So far, more than 40 molecules have been identified to play a critical role in angiogenesis (the process of sprouting of new vessels from pre-existing ones) but most studies to date have focused on vascular endothelial growth factor (VEGF) and its receptors (5). Since 2004, ten drugs that target VEGF or its receptors have been approved for the treatment of various malignant diseases (6). Unfortunately, these agents provide modest survival benefits in some tumor types and have had no efficacy in others. The mechanism underlying this limited antiangiogenic therapy responses are the subject of intense research and will hopefully enable more efficient therapeutic interventions. Nevertheless, numerous studies demonstrate that resistance mechanisms can be regulated by immune cells, which provide a critical source of chemokines and cytokines that promote neovascularization, immunosuppression, and other tumor hallmarks (7,8).
A dysfunctional angiogenic vasculature, such as the one found in many solid tumors, prevents the extravasation of tumor-reactive T cells and fosters an immunosuppressive microenvironment that allows tumors to escape from immunosurveillance. Tumor endothelium for instance can express several inhibitory molecules that can limit the antitumor immune response. The inhibitory checkpoints Programmed cell death ligand 1 (PD-L1) and PD-L2 can be expressed by endothelial cells. Indoleamine 2,3-dioxygenase (IDO1) and T cell immunoglobulin domain and mucin domain protein 3 (TIM3) are expressed by the tumor endothelium (9-11). There is mounting evidence that sustained angiogenesis as well as immunosuppression are interconnected processes which are facilitated by shared regulators in cancer as well as under normal physiological conditions (3). The accumulation of high levels of VEGF in many cancers has been associated with impaired immune cell trafficking and antitumor immunity (3,12). VEGF has been shown to decrease immune cell-endothelial interactions by down-regulating the expression of cell adhesion molecules such as ICAM-1 and VCAM-1 in angiogenic vessels, VEGF also has been shown to directly inhibit dendritic cell (DC) maturation and to activate antigen-specific regulatory T cells (T-reg) (13).
In addition, there is a growing list of haematopoietic cell types that, when appropriately polarized, can promote both immunosuppression and angiogenesis through production of VEGF and other factors such as bFGF, CCL2 and ANGPT2 (3). This list includes tumor-associated macrophages, myeloid-derived suppressor cells, TIE2+ monocytes, immature DCs, and T-reg cells which have been shown to have this dual capacity (3,14-16). A recent study described an involvement of phosphatidylinositol 3-kinase (PI3K) activation in myeloid cells in resistance to antiangiogenic therapy (7) and, more recently, two papers showed the role of a myeloid-specific isoform of PI3K (PI3K gamma) that when inhibited switched the activation state of myeloid cells from an immunosuppressive to a pro-inflammatory phenotype, sensitizing tumors to ICB (17,18).
In this publication by Allen and collaborators (19) they sought to investigate additional immune-related mechanisms of resistance to antiangiogenic therapy and described the effects of combining the inhibition of angiogenesis and ICB. The authors made use of three syngeneic tumor models with different responses to VEGF/VEGFR inhibitors (sorafenib and the anti-VEGFR2 antibody DC101): RIP1-Tag2 pancreatic neuroendocrine tumor model, an MMTV-PyMT mammary carcinoma model and an NFpp10-glioblastoma (GBM) model. Given that Rip-Tag2 mice are refractory to DC101 treatment at about 4 weeks of treatment; it is a good model to evaluate mechanisms associated with therapy resistance. Their findings demonstrate a substantial increase of the immune checkpoint molecule PD-L1 expression, particularly in refractory pancreatic and breast cancer models. The up-regulation of PD-L1 after antiangiogenic treatment, although more pronounced, was not restricted to tumor cells, but also observed in immune cells (CD45+) and endothelial cells (CD31+) in the tumor microenvironment of pancreatic and breast tumors. GBM tumors, although resistant to antiangiogenic therapy, also presented with a modest increase in PD-L1 expression particularly on immune cells. Altogether, Allen et al. findings suggest that PD-L1 upregulation is likely not a mechanism of resistance to antiangiogenic therapy per se, but rather a bystander effect resulting from to the induction of an inflammatory response. It would be interesting to explore effect of antiangiogenic therapy on PD-L1 expression in a tumor-independent context.
Given that PD-L1 is an important interferon (IFN)-responsive gene that is often highly expressed in inflamed tumors (20), the authors investigated whether IFNγ was differentially expressed on CD4+ and CD8+ T cells after antiangiogenic treatment. The rationale is based on preliminary evidences indicating that targeting the tumor vasculature enhances T cell trafficking into the tumor microenvironment (21). Interestingly, treatment increased the frequency of intratumoral IFNγ-expressing T cells as well as Granzyme B-expressing CD8+ T cells in all tumor models, suggesting that an immunostimulatory function is indeed occurring during VEGF/VEGFR blockade. The study would have benefited from functional assays evaluating if the noted changes of Granzyme B and IFNγ expression reflect better effector function of CD8+ T cells in the context of antiangiogenic therapy. Moreover, the authors showed augmented expression of three other IFN-responsive genes (CXCL10, Mx1, Ifit3) in PD-L1+ cells after therapy to support the claim that PD-L1 up-regulation is IFN-mediated. Notably, the most pronounced effects are observed in pancreatic and breast cancer models, and, despite still being present, are very modest in GBM. Given that the data is based on correlation between VEGF/VEGFR and IFN-mediated PDL1 expression the message could be strengthened with the use of IFNγ, IFNγR-KO and/or immunodeficient mouse models to better define a causal link between them.
The authors further examined the combination of VEGF/VEGFR inhibitors with anti-PDL1 therapy and showed notable responses in terms of reduced tumor burden and prolonged survival in pancreatic and breast cancer models, but anti-PDL1 therapy was insufficient to sensitize GBM to antiangiogenic therapy. It is important to note that a number of clinical trials are now evaluating similar combinatory approaches of VEGF/VEGFR and PD-1/PD-L1 inhibition for a number of cancer types such as renal cell carcinoma, colorectal, ovarian cancer and recurrent glioblastoma (NCT03024437, NCT02659384, NCT02873962, NCT02017717), with hopefully promising results for cancer patients as well. Interestingly, the combination with anti-PDL1 reversed the accumulation of PI3K activated myeloid cells (previously associated with both antiangiogenic and immunotherapy resistance) found in anti-VEGFR2 refractory pancreatic as well as breast tumors and GBMs. Moreover, the combination facilitated the infiltration of effector CD8+ T cells and DCs in pancreatic and breast tumors, but did not in therapy-resistant GBMs. The weak stimulation of both innate and adaptive immune cells and lack of significant T cell infiltration after combination treatment could potentially explain the therapeutic resistance observed in GBMs. The presence of tumor-infiltrating T cells is known as a rate-limiting step for therapeutic success in many tumor types. Therefore, it would be interesting to see how tumor-bearing immunodeficient mouse models respond to this combinatorial approach.
Based on the premise that the synergistic effect of antiangiogenic/anti-PD-L1 therapies is immune-dependent, the authors decided to investigate in more detail if alterations of the tumor vasculature and consequently immune cell trafficking could be responsible for the distinct outcomes of antiangiogenic/immunotherapy in the different tumor models. Interestingly, combination therapy stimulated blood vessel normalization—characterized by reduced vessel density and permeability as well as increased pericyte coverage, leading to homogeneous blood flow—in all three tumor types, but it was less prominently in GBMs. Most importantly and possibly as their major finding, the authors demonstrate that morphological changes resembling features of high endothelial venules (HEVs) occur in several areas of the tumor vasculature of pancreatic and breast tumors during combination therapy. HEVs are specialized postcapillary vascular sites, rich in adhesion molecules—such as GlyCAM-1 ICAM-1, CD34 as well as in T cell chemokines such as CCL21—that mediate T cell adherence and transendothelial migration into secondary lymphoid organs (22). The authors provide confirmatory data, by immunohistochemical analysis using a HEV-specific MECA79 antibody, that antiangiogenic/anti-PD-L1 therapy induces the formation of HEVs only in pancreatic and breast tumors, but not in the resistant GBMs. Also, the MECA79 + HEV tumor vessels had an increased number of T and B cells, which is a common feature of normal HEVs. All together, their findings suggest that the beneficial effects of the combination therapy are likely dependent on intratumoral HEV formation, which consequently facilitates T-cell trafficking (Figure 1).
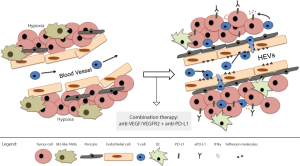
In their final results, the authors present some interesting data showing the induction of several lymphotoxin β receptor (LTβR)-regulated genes, including its ligand LIGHT, in HEV+ tumors upon antiangiogenic/anti-PD-L1 therapy. Lymphotoxin and LIGHT, known inducers of LTβR, are expressed by a number of activated immune cells such as DCs, Natural Killer cells, B and T cells (23,24) They proceeded to test whether stimulating LTβR signaling directly with the use of agonists or antagonists could promote HEV formation. Indeed, LTβR-activation alone and in combination with antiangiogenic/immunotherapy implicated in the formation of HEVs, increased lymphocytic infiltration and tumor protection in all tumor models, including GBMs. Further studies aiming to understand the biology behind the formation of intratumoral HEVs as well as the therapeutic potential of LTβ inducers, such as LIGHT, are needed and will certainly enable the development of novel targets and treatment options for cancer patients.
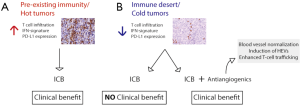
In summary, overcoming immunotherapy resistance is a major focus of research in the scientific community and the identification of mechanisms based on means to facilitate blood vessel normalization and enhanced T cell trafficking has important implications on the design of more effective rationally designed combinatorial strategies. Also, understanding what makes certain tumors, such as GBMs in this study, intrinsically more resistant to the formation of HEVs and antiangiogenic/immunotherapy combination is a promising avenue of research. Successful immunotherapy must offer rational combinatorial strategies that take into consideration tumor features in a personalized manner, in order to effectively overcome resistance mechanisms (Figure 2). Switching “cold” into “hot” tumors, based on tumor T-cell infiltration and inflammatory gene-expression signature, is an attractive modality, given the requirement of T cell infiltration in different therapeutic modalities for efficient eradication of tumor cells. The findings presented by Allen et al. and Schmittnaegel et al. (19,25), published in the same edition, move forward in that direction and introduce a proof of concept for exploring therapies targeting the tumor vasculature to enhance T cell trafficking and to sensitize tumors which are resistant to ICB.
Acknowledgements
We would like to thank Swim Across America, Ludwig Institute for Cancer Research, Parker Institute for Cancer Immunotherapy and the MSKCC Core Grant (P30 CA008748) for their suport. We would also like to thank Sadna Budhu and Aditi Gupta for their critical review of this manuscript.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Khalil DN, Smith EL, Brentjens RJ, et al. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol 2016;13:394. [Crossref] [PubMed]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature 2000;407:249-57. [Crossref] [PubMed]
- Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol 2011;11:702-11. [Crossref] [PubMed]
- Nagy JA, Chang S-H, Dvorak AM, et al. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer 2009;100:865-9. [Crossref] [PubMed]
- Jayson GC, Kerbel R, Ellis LM, et al. Antiangiogenic therapy in oncology: current status and future directions. Lancet 2016;388:518-29. [Crossref] [PubMed]
- Jain RK. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell 2014;26:605-22. [Crossref] [PubMed]
- Rivera LB, Meyronet D, Hervieu V, et al. Intratumoral Myeloid Cells Regulate Responsiveness and Resistance to Antiangiogenic Therapy. Cell Rep 2015;11:577-91. [Crossref] [PubMed]
- Shojaei F, Wu X, Malik AK, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol 2007;25:911-20. [Crossref] [PubMed]
- Huang X, Bai X, Cao Y, et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J Exp Med 2010;207:505-20. [Crossref] [PubMed]
- Riesenberg R, Weiler C, Spring O, et al. Expression of indoleamine 2,3-dioxygenase in tumor endothelial cells correlates with long-term survival of patients with renal cell carcinoma. Clin Cancer Res 2007;13:6993-7002. [Crossref] [PubMed]
- Rodig N, Ryan T, Allen JA, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol 2003;33:3117-26. [Crossref] [PubMed]
- Dirkx AE, Oude Egbrink MG, Kuijpers MJ, et al. Tumor angiogenesis modulates leukocyte-vessel wall interactions in Vivo by reducing endothelial adhesion molecule expression. Cancer Res 2003;63:2322-9. [PubMed]
- Gabrilovich D, Ishida T, Oyama T, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998;92:4150-66. [PubMed]
- Yang L, DeBusk LM, Fukuda K, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004;6:409-21. [Crossref] [PubMed]
- Riboldi E, Musso T, Moroni E, et al. Cutting edge: proangiogenic properties of alternatively activated dendritic cells. J Immunol 2005;175:2788-92. [Crossref] [PubMed]
- Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 2011;475:226-30. [Crossref] [PubMed]
- De Henau O, Rausch M, Winkler D, et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 2016;539:443-7. [Crossref] [PubMed]
- Kaneda MM, Messer KS, Ralainirina N, et al. PI3Kgamma is a molecular switch that controls immune suppression. Nature 2016;539:437-42. [Crossref] [PubMed]
- Allen E, Jabouille A, Rivera LB, et al. Combined antiangiogenic and anti–PD-L1 therapy stimulates tumor immunity through HEV formation. Sci Transl Med 2017;9:eaak9679. [Crossref] [PubMed]
- Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. [Crossref] [PubMed]
- Lanitis E, Irving M, Coukos G. Targeting the tumor vasculature to enhance T cell activity. Curr Opin Immunol 2015;33:55-63. [Crossref] [PubMed]
- Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today 1995;16:449-57. [Crossref] [PubMed]
- Wang Y, Zhu M, Miller M, et al. Immunoregulation by tumor necrosis factor superfamily member LIGHT. Immunol Rev 2009;229:232-43. [Crossref] [PubMed]
- Girard JP, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol 2012;12:762-73. [Crossref] [PubMed]
- Schmittnaegel M, Rigamonti N, Kadioglu E, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci Transl Med 2017;9:eaak9670. [Crossref] [PubMed]


