Double conduction through the atrioventricular node following acute medullary infarction: a case report
Introduction
Lateral medullary syndrome (LMS), also known as Wallenberg’s syndrome, PICA syndrome, results from occlusion of the posterior inferior cerebellar artery, with associated infarction of parts of the medulla oblongata, and cerebellum on the ipsilateral side. It often manifests with various patterns of sensory, motor, and autonomic deficits. While sensorimotor dysfunction presents as a predicted pattern of clinical signs and symptoms, autonomic dysfunction is usually less clinically apparent and can be easily mistaken as a concomitant pathology in the end organ it affects. In this case, we present a case of an unusual pattern of cardiac arrhythmia as the first objective finding of LMS, caused by autonomic instability following infarction of the vagus nerve nuclei in the medulla (1).
Case presentation
A 59-year-old Caucasian man with past medical history of essential hypertension presented to the emergency room (ER) with vertigo of 2-week duration. His vertigo was episodic, lasting for a few minutes then subsiding, and improving with lying supine with his eyes closed. The vertigo slightly improved over the course of 2 weeks. However, one day prior to presentation, it became more severe, longer lasting, and not relieved by lying supine, with associated nausea. Progression of vertigo seemed to be sudden rather than gradual. A complete review of systems was performed and was otherwise unremarkable.
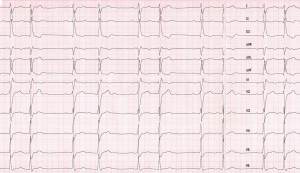
In the ER, his blood was 162/95 mmHg; with a heart rate recorded as 31 beats per minute. Physical examination was reported to be otherwise normal. A 12-lead electrocardiogram was obtained and showed regularly irregular bradyarrhythmia (Figure 1). Initial laboratory and radiographic work up, including complete blood count, electrolytes and chemistry, cardiac biomarkers, chest X-ray were all within normal limits. Urgent transvenous pacing and transfer to the coronary care unit were initially considered, as symptomatic bradycardia was thought be responsible for his neurological symptoms. This was deferred as the patient was stable, and there were no signs of tissue hypoperfusion and blood pressure was elevated.
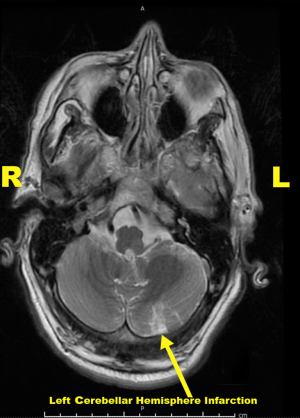
Neurology and cardiology consultations were sought. Detailed neurological exam at this time revealed minor unilateral facial paralysis, manifested as mildly flattened and asymmetric nasolabial fold on the left, and bilateral vertical and horizontal nystagmus with left arm dysmetria. National Institutes of Health Stroke Scale (NIHSS) was 2. CT scan of the head was negative for radiographic evidence of ischemia, infarction or any other pathology, and CT angiograms of the head and neck were also unremarkable. A brain MRI was performed and showed acute ischemic infarct of the left lateral medulla and subacute infarct of the inferolateral cerebellar hemisphere on the left (Figure 2). Both lesions are in the territory of the left posterior inferior cerebellar artery. This radiographic description, along with history and physical exam findings, were diagnostic for LMS. The patient presented outside the recommended window for thrombolytic therapy. Hence, he was treated according to subacute stroke treatment guidelines with high intensity statin, aspirin, and aggressive blood pressure control. He made good recovery with improvement of his neurological symptoms. His arrhythmia has resolved on its own within the first 24 hours after presentation without any further intervention. He was discharged after few days from the hospital with home physical therapy visits.
EKG interpretation
Even though the primary diagnosis was (LMS) in this patient, deeper understanding of anatomic and electrophysiological characteristics of the atrioventricular (AV) node is essential to explain the unusual presenting bradyarrhythmia. The first striking objective finding in this case to be highlighted is the abnormal electrocardiogram. It shows a regularly irregular narrow complex monomorphic bradyarrhythmia with clustering of QRS complexes that appear as couplets. This rhythm led to an erroneously low recorded heart rate by the ER triage room monitor. EKG interpretation in this case is challenging due to its unusual pattern. The underlying rhythm is regular atrial sinus bradycardia. All P waves have the same morphology, as they all originate from the sinoatrial node (SA node). P to P interval is 1,200 ms, which reflects an underlying sinus rate of 50 beats per minute. Every other QRS complex (odd numbered, i.e., 1, 3, 5 etc.) is preceded by a P wave with P-R interval of 260 ms. Prolonged P-R interval is seen before every other QRS complex, in a pattern consistent with first degree AV block. The remaining, even numbered QRS complexes appear identical in morphology to the odd numbered QRS complexes, but they conduct significantly closer to preceding QRS complexes, and farther from the following ones, giving the appearance of regularly irregular couplets (Figure 3). These “unique” QRS complexes are not preceded nor followed by P waves. Instead, they contain the regular sinus P waves that are buried within their waveform of these unique QRS complexes, or immediately thereafter, in the following ST segment.
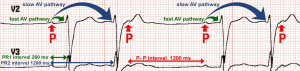
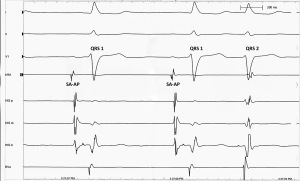
These unique QRS complexes seem to conduct 1,200 ms after the P wave that initiated the preceding QRS complex. In other words, every other sinus P wave seems to initiate two QRS complexes with two different P-R conduction intervals; one after 260 ms (PR-1), followed by another after 1,200 ms (PR-2). This interesting pattern of double sequential conduction of a single sinus action potential is attributed to the electrophysiological properties of the AV node in this patient; dual “slow” and “fast” AV conduction pathways. It is often termed: dual firing pattern, or 2 for 1 conduction. The patient had an electrophysiological study during the same hospitalization, confirming the presence of dual AV nodal pathways with fast and slow conduction (Figure 4). Previous EKGs obtained on different hospitalizations were reviewed, and it was determined that this is a new conduction abnormality. It seems that autonomic dysfunction following medullary infarction unmasked the dual pathway system of the AV node, presenting with this conduction abnormality.
Discussion
The brainstem is a very complex structure. It is the bottleneck of the central nervous system that harbors many neurological ascending and descending motor and sensory pathways. It also contains many vital clusters of densely packed cell bodies of neurons, called “nuclei”. The medulla oblongata contains key nuclei involved in autonomic control, including the nucleus tractus solitarius (NTS), the dorsal vagal nucleus, the nucleus ambiguous and the intermediate reticular zone (2).
The AV node is also a structurally complex segment of the cardiac conduction system. In previous human experiments, it has been shown that some individuals have dual functional AV node pathways, each with different speed, timing, consistency and direction of conductivity. It has not been proven, however, that any special structural properties of the AV node, makes it a substrate for dual AV nodal pathways (3).
LMS is caused by a brainstem infarction as a sequela of vascular event in the territory of the posterior inferior cerebellar artery or the vertebral artery. Acute thrombosis or thromboembolism to this vascular territory manifests as motor, sensory, or autonomic deficits. The dorsal vagal nucleus and the NTS are part of the autonomic nervous system. They play a key role in cardiovascular autonomic regulation. LMS may cause acute infarction of these two nuclei or their proprietary pathways, resulting in alteration of parasympathetic and sympathetic outflow to the heart (4).
Extensive literature review showed very few case reports of cardiac arrest, sinus bradycardia, or sinus pauses following acute medullary infarction (5). There have not been any published cases of dual AV node conduction pathways with different conduction speeds that were unmasked by LMS. It seems that autonomic cardiac dysfunction after medullary infarction is an uncommon, but potentially life threatening complication. This case report adds a new, unrecognized manifestation of autonomic dysfunction pertinent to the heart following medullary infarction.
Conclusions
Arrhythmias are commonly associated with structural or conduction disorders of the myocardium. However, disturbance of the heart rhythm could also be due to pathology affecting other organ systems. Assessment of arrhythmia should always incorporate full history and physical examination, taking into account that non-cardiac signs and symptoms may highlight the underlying diagnosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Lee MJ, Park YG, Kim SJ, et al. Characteristics of stroke mechanisms in patients with medullary infarction. Eur J Neurol 2012;19:1433-9. [Crossref] [PubMed]
- De Caro R, Parenti A, Montisci M, et al. Solitary tract nuclei in acute heart failure. Stroke 2000;31:1187-93. [Crossref] [PubMed]
- Lee KL, Chun HM, Liem LB, et al. Multiple atrioventricular nodal pathways in humans: electrophysiologic demonstration and characterization. J Cardiovasc Electrophysiol 1998;9:129-40. [Crossref] [PubMed]
- Koay S, Dewan B. An unexpected Holter monitor result: multiple sinus arrests in a patient with lateral medullary syndrome. BMJ Case Rep 2013;2013:bcr2012007783. [Crossref] [PubMed]
- von Heinemann P, Grauer O, Schuierer G, et al. Recurrent cardiac arrest caused by lateral medulla oblongata infarction. BMJ Case Rep 2009;2019:bcr02.2009.1625.