Treating ALK-positive non-small cell lung cancer
Background
The identification of rearrangements in the anaplastic lymphoma kinase (ALK) gene in patients with non-small cell lung cancer (NSCLC) and the rapid development of effective ALK-directed tyrosine kinase inhibitors (TKI) represent a modern example of personalized treatment for advanced lung cancer (1,2). The initial enthusiasm coming from the favorable outcomes of crizotinib, the first oral ALK TKI approved for the treatment of ALK-positive NSCLC, was slow down, since almost all treated patients unavoidably developed resistance within approximately 12 months and experienced disease progression, mainly in the brain or in other parenchymal sites (3). Second generation ALK TKIs, including Ceritinib (LDK378), Alectinib (CH5424802/RO5424802) and Brigatinib (AP26113) were developed in order to overcome the acquired resistance to therapy and to improve efficacy in crizotinib-pretreated ALK-positive patients, even in those with metastases to central nervous system (CNS) (4,5). Up to date, crizotinib, ceritinib, alectinib and brigatinib have received approval from the Food and Drug Administration (FDA) and/or the European Medicines Agency (EMA) for the treatment of ALK-rearranged NSCLC. In addition, ongoing clinical trials demonstrated that 3rd-generation ALK TKIs, including Lorlatinib (PF-06463922), Entrectinib (RxDx-101) and Ensartinib (X-398) provided promising preliminary data in terms of both clinical activity and safety (6).
In this review, we will further focus on the fundamental role of ALK gene rearrangements in NSCLC, the different biology of this molecular subtype of lung cancer, the optimal methodology for the diagnosis of ALK positivity, and we will follow the entire path from the pre-clinical data to the established phase III clinical trials of all available ALK-directed therapeutic options, beginning from crizotinib up to current investigational ALK-inhibitors. At the end, we will discuss all the raised issues from their use in clinical practice as well as our considerations about the optimal frontline approach and the exact sequence of ALK inhibitors in the treatment of ALK (+) NSCLC.
Discovering the underlying biology of ALK-rearranged NSCLC
The ALK gene, located on chromosome 2p23, encodes a protein belonging to the family of the insulin-receptor tyrosine kinases that activates multiple downstream pathways (6-8). ALK gene was initially described in 1994 as it was involved in a translocation with the gene encoding for nucleophosmin between chromosome 2 and 5 [t(2,5)(p23;q35)] in anaplastic large cell lymphoma (9). The native form of ALK protein (1,620 amino-acids) consists of an extracellular domain with the two ligands, a single-chain transmembrane segment and an intracellular domain (1,10,11). The ALK protein is apparently inactive and is not expressed in the normal lung tissue (12). Only during embryogenesis as demonstrated in mice models, ALK is highly expressed in the brain and peripheral nervous system (13,14). Various breakpoints in the context of ALK gene causes the formation of different variants which are merged with a fusion partner such as kinesin family member-5B (K1F5B), kinesin light chain-1 (KLC1), transforming growth factor (TGF), Translocated Promoter Region (TPR), huntingtin interacting protein-1 (HIP1), striatin (STRN), dynactin subunit-1 (DCTN1), sequestosome-1 (SQSTM1) and baculoviral IAP repeat containing-6 (BIRC6), resulting in the aberrant expression of ALK-translocated compound (15,16). The most frequent ALK translocation usually involves the gene of echinoderm microtubule-associated protein-like 4 (EML-4) and was firstly identified in 2007 in a 62-year-old male patient with lung adenocarcinoma (17). After dimerization, EML4-ALK transcript constitutively drives the activation of mitogen-activated protein kinase (MAPK), Janus kinase with signal transducer and activator of transcription (JAK-STAT) and phosphoinositide-3-kinase with Akt murine thymoma viral oncogene homolog (PI3K-AKT), leading to an increase in proliferation and survival and boosting the angiogenetic switch in cancer cells (7,18). Oncogenic activation of ALK pathway has been also identified in other malignancies including neuroblastoma, inflammatory myofibroblastic tumours, diffuse large B-cell lymphoma, breast cancer, oesophageal squamous cell carcinoma, and colorectal cancer (via fusions with other gene partners) (17-21). In NSCLC, rearrangements of ALK gene were recognized in approximately 3–7% of patients with higher rates observed in a clinically enriched population (younger, never or light smokers) of adenocarcinoma patients (22-24). Regardless the personal characteristics such as gender, race and smoking status, testing for the identification of rearranged ALK is recommended in all patients with lung adenocarcinoma (or where adenocarcinoma cannot be excluded).
Diagnosis of ALK (+ ) NSCLC: methods for detecting EML4-ALK rearrangements
The rarity of ALK genetic alternations in contrast to the high incidence of NSCLC, predispose for an optimal, accurate, cost-effective, fixed-time testing that fits with the other established diagnostics for identification of epidermal growth factor receptor (EGFR) mutations. However, several different methods including fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), and reverse transcription-polymerase chain reaction (RT-PCR) have been developed in order to detect ALK-rearranged NSCLC, each one with advantages and disadvantages (25,26). The Vysis Dual color break-apart FISH (Abbott Molecular, Des Plaines, IL, USA) is FDA approved for the diagnosis of patients with ALK-positive NSCLC. Among them, it is quite difficult to recognize a comprehensive methodology as these tests are complimentary but not mutually exclusive and their use depends on local availability, expertise and resources.
Crizotinib
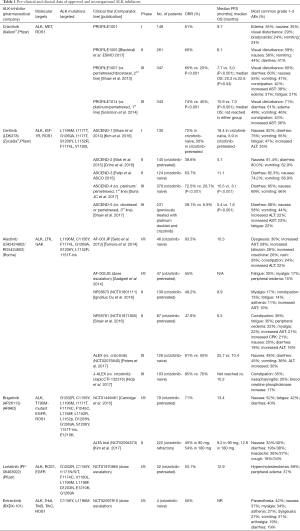
Crizotinib is the first oral ALK TKI approved by the FDA for the treatment of ALK-rearranged NSCLC in August 2011, just 4 years after the first identification of ALK-rearranged NSCLC. Crizotinib exhibits an additional inhibitory activity against other kinases including c-MET or Hepatocyte Growth Factor Receptor (HGFR) protein-tyrosine kinase (27), as well as ROS1 kinase that shares 77% of amino-acid sequences of ATP-binding sites with the ALK (28). Working as an ATP-competitive molecule, crizotinib inhibits sustainably ROS1 kinase function, providing an overall response rate (ORR) of 72% in patients with NSCLC harboring the translocation of ROS1 (29). This ROS1 inhibition induced by crizotinib supports its FDA approval also for ROS1-rearranged NSCLC on 2016 and the further clinical applications of the drug in other ROS1-positive tumors. Table 1 summarizes the molecular targets and the main outcomes and toxicities of Phase I, II and III clinical trials of crizotinib. The initial phase I PROFILE 1001 trial exploring the pharmacokinetic profile of crizotinib in patients with ALK-positive NSCLC identified a dose of 250 mg twice daily (BID) as the recommended dose for phase II studies (4). At that dose, the ORR was 61%, with the median progression-free survival (PFS) of 9.7 months (4). The PROFILE 1005 trial reported in the last ESMO 2017 was the largest study to date for any ALK inhibitor in ALK-positive advanced NSCLC and provides further data from over 1,000 patients supporting the clinical efficacy and safety profile of crizotinib in patients with previously treated ALK-positive NSCLC. The PROFILE 1005 results confirmed an ORR of 60% and a median PFS of 8.1 months with a good tolerability. The preliminary results of PROFILE 1001 and 1005 trials led to the fast track approval of crizotinib for second or further treatment lines of ALK-rearranged NSCLC patients, supporting the development of phase III trials. The PROFILE 1007 trial was the first phase III trial comparing crizotinib to standard second-line chemotherapy (docetaxel or pemetrexed), in patients with advanced ALK-rearranged NSCLC, progressing after a 1st-line platinum-based chemotherapy (30). Randomizing 347 patients, this study showed a significantly longer median PFS (7.7 vs. 3 months, P<0.001) and a significantly higher ORR (65% versus 20%; P<0.001) for crizotinib compared to chemotherapy. Due to the high crossover rate, no difference in OS was reported between the two treatment arms, with a median OS of 20.3 versus 22.8 months (30). In the subgroup analysis of PROFILE 1007, crizotinib-treated patients with brain metastases did not retain any significant PFS benefit compared to chemotherapy, suggesting a low penetration of the TKI via the brain-blood barrier (30). Next, the PROFILE 1014, a phase III, open-label, randomized study, evaluated crizotinib in 1st line setting, versus platinum-based doublet in patients with advanced ALK-positive non-squamous NSCLC who had not received prior systematic therapy (31). Crizotinib provided a significantly prolonged PFS (10.9 vs. 7.4 months, P<0.0001), and ORR (74% vs. 45%, P<0.001). Similarly to PROFILE 1007, the analysis of OS was affected by the high crossover rate (70% of patients in chemotherapy arm) and median OS was not reached in either group (31). In the PROFILE 1007 and the PROFILE 1014 56% and 70%, respectively continued crizotinib beyond progression (30,31). A retrospective approach of the two single arms of these trials showed that patients achieving a clinical benefit from crizotinib maintain their good performance status and survive longer if they continued the drug instead of stopping it at RECIST disease progression (16.4 vs. 3.9 months; P<0.0001) (32).
Full table
Ceritinib (LDK378)
Ceritinib is a 2nd-generation, ATP competitive, highly selective ALK inhibitor (20 times more potent than crizotinib) and a potent inhibitor of IGFR-1 but not efficient inhibitor of c-MET (33,34). Preclinical data showed that ceritinib was active against many ALK mutations that conferred resistance to crizotinib, including C1156Y, F1174C, G1202R and the gatekeeper mutation L1196M but also in crizotinib-resistant lines without known resistance mutations to crizotinib (34). In the landscape of clinical trials, the phase I ASCEND-1 study began from a dose-escalation part and reached to an expansion cohort, including both crizotinib-naïve and crizotinib pre-treated patients with ALK-rearranged NSCLC (35,36). The dose of ceritinib was escalated from 50 to the maximum tolerated dose (MTD) of 750 mg per day and the dose-limiting toxic events included diarrhea, vomiting, dehydration, elevated aminotransferase levels, and hypophosphatemia. Among patients with NSCLC who received a daily dose of 400 mg or higher, the ORR was 58% and the median PFS was 7 months. In crizotinib-naïve patients, the ORR was 62% and the PFS 10.4 months, whereas in the crizotinib-resistant patients the ORR was 56% and the median PFS 6.9 months (35). In the expansion cohort of ASCEND-1 after additional accrual (n=246, 163 crizotinib-pretreated and 83 crizotinib-naïve patients), ORR in the overall population reached to 61.8% and median PFS to 9 months (36). In crizotinib-naïve and pretreated patients, the updated median PFS was 18.5 and 6.9 months, respectively (36). Based on these results, the FDA approved ceritinib for patients with advanced/metastatic ALK-positive NSCLC progressing to crizotinib. In Europe, the EMA recommended granting a conditional marketing authorization for ceritinib in patients with ALK-positive NSCLC previously treated with crizotinib (35,36). Two phase II single-arm studies on ceritinib in patients with ALK (+) NSCLC were presented: the ASCEND-2 trial (37) in patients pretreated with at least one prior chemotherapy regimen (including platinum-based doublet) and had progressed on crizotinib as the last therapy, and the ASCEND-3 trial in crizotinib-naive patients. The ASCEND-2 study enrolled 140 patients, 71.4% with brain metastases, 28% of whom had no prior brain radiation, documented an ORR of 38.6% and a median PFS of 5.7 months (37). At baseline, 20 patients had measurable CNS disease and their intracranial overall response rate (IC-ORR) was 45%. Felip and colleagues presented the preliminary results of ASCEND-3 trial at ASCO meeting 2015. Among 124 enrolled patients, 40.3% with brain metastases, 46% of whom had no prior BRT, documented an ORR of 63.7%. At baseline, 10 patients had investigator-assessed measurable CNS disease and the IC-ORR was 20%. In both studies, the most common grade 1–2 AEs were diarrhea (80% and 82.3%), nausea (81.4% and 74.2%), and vomiting (62.9% and 66.9%), while 7.3% and 7.9% patients discontinued treatment due to AEs, none of which were predominant. A large retrospective analysis showed a median PFS with sequential crizotinib and ceritinib of around 18 months, supporting the idea that continuous targeting and inhibition of ALK pathway might represent a promising therapeutical strategy (38).
Although that crizotinib had already been proven superior to chemotherapy changing the best control arm, two additional randomized phase III trials completed their comparison of ceritinib with standard chemotherapy in first-line and 3rd-line setting (ASCEND-4 and ASCEND-5) (39,40). The ASCEND-4 trial showed a statistically significant and clinically meaningful improvement in outcomes of ceritinib compared to platinum-pemetrexed doublet and pemetrexed maintenance in untreated patients with advanced ALK-rearranged NSCLC (39). Median PFS was 16.6 vs. 8.1 months (P<0.001) and overall as well as intracranial response rate were 73% vs. 27%, respectively in the two therapeutic arms. The ASCEND-5 trial compared ceritinib with docetaxel or pemetrexed in 231 patients with ALK-rearranged NSCLC who had previously received a platinum doublet and crizotinib and had subsequent disease progression (40). The primary outcome of PFS was 5.4 months with ceritinib and 1.6 months with chemotherapy (P<0.001) and ORR with ceritinib (39.1%) exceeded that of chemotherapy (6.9%). Based on the aforementioned ASCEND trials, the safety profile of ceritinib included mainly gastrointestinal AEs (Table 1). As it will be discuss below, the control of brain metastases was better with ceritinib than with chemotherapy and the patient-reported outcomes are less rapidly deteriorated in patients receiving ceritinib than chemotherapy (40).
Alectinib (RO5424802/CH5424802)
Alectinib is an oral highly selective ALK TKI active both in crizotinib-naïve and in crizotinib-resistant ALK-rearranged NSCLC cases. Preclinical data revealed that it targets several ALK mutations that confer resistance to crizotinib (L1196, the gatekeeper mutation in crizotinib-resistant mutants, as well as C1156Y, F1174L, R1275Q, and G1269A), but not ROS1 and IGF-1R (41-43). In contrast to crizotinib, alectinib penetrates to CNS, as it is not a substrate of P-glycoprotein (P-gp), a key efflux transporter located at the blood-brain barrier (44-46). In June 2013, alectinib was granted by the FDA for patients with ALK (+) NSCLC who progressed on crizotinib and in July 2014 Japan firstly approved alectinib in previously untreated patients with advanced ALK-rearranged NSCLC, based on the results of the phase I/II AF-001JP trial (47). Afterward in 2015, FDA approved alectinib in the USA, for crizotinib-resistant patients with ALK-positive NSCLC and this year [2017] alectinib receives EU approval for patients with previously treated ALK-positive NSCLC. Starting from the AF-001JP trial, phase I part selected as recommended a dose of 300 mg BID and the phase II arm reported an ORR of 93.5% in 46 patients treated at 300 mg BID (47). However, a second phase I/II study (NCT01588028) in crizotinib pre-treated Western patients with ALK (+) NSCLC increased the MTD to 600 mg BID and subsequent results from the phase II part of study reported ORR in 55% of 44 patients and intracranial responses in 52% of the 21 patients with measurable CNS metastases (45). This dose has been further evaluated in two single-arm phase II trials, one including 87 patients from North America (NCT01871805) (48), and the other 138 patients from 16 different countries (NCT01801111) (46) whose disease progressed on crizotinib. Treatment with alectinib resulted in ORRs of 47.8% and 49.2%; median PFS was 6.3 and 8.9 months; duration of response continued for a median of 7.5 and 11.2 months, and intracranial response for patients with baseline CNS disease were 57% and 75%, respectively (46,48). Comparing ALK inhibitors as first-line options, both randomized phase III trials, the Japan J-ALEX (JapicCTI-132316) and the global ALEX trial (NCT02075840), showed superiority of alectinib over crizotinib for the management of ALK-positive advanced NSCLC (49,50). In the first head-to-head comparison, J-ALEX trial showed a favorable PFS for alectinib (median PFS: not reached) compared with crizotinib (median PFS: 10.2 months) (50). Grade ≥3 AEs occurred at a greater frequency with crizotinib (52%) than alectinib (26%) while dose interruptions/discontinuations due to toxicities were also more commonly observed with crizotinib (74%/20%) than with alectinib (29%/9%) (50). In the global ALEX trial, 41% of patients in the alectinib group versus 68% in the crizotinib group experienced a disease progression or death during follow-up (49). The rate of one-year PFS was significantly higher with alectinib than with crizotinib (68.4% vs. 48.7%; P<0.001) while the median PFS with alectinib was not reached. Only 12% in the alectinib group had an event of CNS progression, as compared with 45% in the crizotinib group (P<0.001). However, the ORR in the alectinib group and in the crizotinib group (82.9% and 75.5%, respectively) did not reached to significant difference (P=0.09). Grade ≥3 AEs were less frequent with alectinib (41% vs. 50%) but not significantly compared to crizotinib (49). These results showed that alectinib has both systemic efficacy and intracranial disease control in patients with ALK-positive NSCLC and brain metastases, supporting a potential change of first-line option for ALK inhibition. As a subsequent line option, the comparison of alectinib with pemetrexed in patients with ALK-positive NSCLC previously treated with platinum-based chemotherapy and crizotinib is under investigation by a phase III trial (NCT02604342).
Brigatinib (AP26113)
Brigatinib is developed as an oral TKI with great activity against many ALK secondary mutations including the gatekeeper mutation L1196M and the G1202R mutation that mediates acquired resistance to other ALK TKIs. In addition, it has lower potency against, ROS1, IGF-R1, insulin receptor kinase, FLT3 and T790M-mutant EGFR (51,52). Due to this complex inhibitory mechanism, brigatinib could represent a promising therapeutic option for patients progressing to crizotinib due to activation of the EGFR bypass pathway (5). In molecular basis, it is a dimethylphosphine oxide group-containing TKI constructed around a bisanilinopyrimidine scaffold. Zhang et al. have shown that the plasma levels of brigatinib achieved at a dose of 180 mg exceeded a 90% inhibition of cell viability for all ALK mutants while at a dose of 90 mg exceeded a 90% inhibition of cell viability for all ALK mutants except of G1202R which is the most refractory of ALK mutants (52). The antitumor activity and safety profile of brigatinib were evaluated in a phase I/II trial. In the phase 1 part performed in patients with advanced malignancies the recommended phase 2 dose was determined to be 180 mg daily. Both treatment-naïve and crizotinib-resistant patients with ALK-positive NSCLC were enrolled independently on CNS involvement. In patients with crizotinib-resistant disease, the ORR was 71%, median PFS was 13.4 months and DOR was 9.9 months. In the small group of crizotinib-naïve patients, the ORR was 100%, while median PFS and DOR have not yet been reached (53). Among the patients with measurable brain disease, the IC-ORR was 53%. Among patients with no measurable brain disease, the IC-ORR was 33%. In the group of patients who had not received prior brain radiotherapy, the IC-ORR was 56% for those with measurable disease and 50% for those without. However, treatment-related serious pulmonary AEs were occurred including dyspnea (7%) pneumonia (7%) and hypoxia (5%), within 7 days of treatment initiation or re-initiation following a prolonged period of dose interruption. These AEs were managed with dose interruption and empiric treatment with steroids and antibiotics. The rates of these AEs were lower with lower starting doses. Given the concern for this early pulmonary toxicity at 180 mg, a lead-in dose of 90 mg daily for a week was pursued. Recently published results of ALTA (54) reported that patients with ALK-rearranged NSCLC who received brigatinib after crizotinib, could achieve substantial overall and intracranial, responses as well as robust PFS. The phase II ALTA trial randomized 222 patients with ALK (+) NSCLC pretreated with crizotinib in two different doses of brigatinib, 90 and 180 mg once daily. The 180 mg (with lead-in dose of 90 mg) showed consistently better efficacy than 90 mg, (ORR was 45% and 54% and the median PFS was 9.2 and 12.9 months, respectively) with acceptable safety. Among patients with measurable brain disease at baseline the IC-ORR was 36% at 90 mg and 67% at 180 mg (54). Early onset (within the first 7 days) pulmonary toxicity occurred only in the 6% of patients (3% were grade 3–4) and was correlated to the higher dose of the drug (54). In October 2014, brigatinib received a breakthrough therapy designation from the FDA for the treatment of patients with ALK (+) crizotinib-resistant NSCLC, based on the findings of ALTA trial. The drug is currently under evaluation in both first-line setting versus crizotinib in a phase III trial (ALTA-1L, NCT02737501) and in phase II study (NCT02706626) after failure of other second-generation ALK inhibitors (ceritinib and alectinib). This last study aims to explore the possibility for brigatinib to overcome the resistance to ALK inhibitors mediated by potent secondary ALK mutations such as G1202R and I117N/S/T100.
Lorlatinib (PF-06463922)
Lorlatinib is a novel, selective ATP-competitive brain-penetrant inhibitor of ALK and ROS1, with clinical activity against all the recognized mutations driving resistance to crizotinib, ceritinib and alectinib (including the G1202R mutation) (55). Its antitumor efficacy initially detected in vitro and in xenograft models harboring crizotinib-resistance mutations (1,56). At the recommended dose of 100 mg/day established by a dose escalation phase I/II trial of Bauer et al. presenting at ASCO meeting 2015 (57), the ORR was 46% regardless of the number of prior ALK inhibitors. In this study, a patient with double resistance to crizotinib and ceritinib achieved a PFS of 8 months with lorlatinib while the secondary mutation of L1198F drived to subsequent resistance to lorlatinib. Interestingly the patient was re-sensitized to crizotinib after lorlatinib failure, indicating that retreatment under molecular guidance should be considered a clinically meaningful approach in ALK-translocated NSCLC (58). Regarding safety, the most common all grade AE reported in the phase I study of Bauer et al. was hypercholesterolemia (65% all grade, 10% grade 3–4) and neurologic/psychiatric disorders affecting the state of consciousness in the 30–35% of patients, however all of them occurred at low grade. Lorlatinib has not yet been approved by any regulatory agency.
Entrectinib (RXDX-101) and Ensartinib (X-396)
Entrectinib (RXDX-101) was developed as an ALK inhibitor with potent activity against crizotinib-mediated resistance mutations, including L1196M and CC1156Y. Entrectinib acts also as a pan-TRK inhibitor, inhibiting NTRK1-3 fusion proteins, an emerging target in oncogene addicted NSCLC (59). Two cohorts, the Italian ALKA 372-001 and the international STARTRK-1A of NRTKROS1-ALK rearranged patients were included in a phase I trial in order to establish the dose of 600 mg/daily entrectinib in a continuous schedule as the recommended dose for further studies (1). In 24 patients previously untreated with targeted therapies, the ORR was 57% for ALK-rearranged cancers, 100% for NRTK1-3 fusion cancers and 85% for ROS1-translocated cancers. Regarding safety, paresthesia and fatigue were the commonest toxicities in the two cohorts. The activity of entrectinib is still under evaluation by a phase II basket study, the STARTRK-2 trial (NCT02568267) in patients with ALK, ROS1 or NRTK1-3 rearrangement previously untreated with targeted therapies.
Ensartinib (X-396) is a novel ALK inhibitor with additional activity against MET, ABL, AXL, ROS1. Preliminary results of a recent phase I trial presenting in an abstract form showed an ORR of 60% and of 88% in crizotinib-naïve and crizotinib-resistant patients, respectively, at a dose of 225 mg/day. As published in the last ASCO of 2016 this study found a decrease in ALK gene expression in responders through serial plasma sequencing of enrolled patients, suggesting it as clinical tool for monitoring of response and of acquired resistance.
Upcoming ALK inhibitors
Following the rationale of add-on therapies to a future best-in-class ALK TKI and beside to lorlatinib, entrectinib (RXDX-101) and ensartinib (X-396) that have not received yet any approval, additional next-generation ALK inhibitors, including Belizatinib (TSR-011), ASP3026, TPX-0005, F17752, CEP-37440, CEP-28122 and GSK1838705A are under investigation. These ALK inhibitors are expecting to enhance more anti-ALK activity, to overcome or delay development of high-grade resistance mutations and to improve the control of CNS disease. Targeting continuously ALK inhibition could preserve quality of life of patients with advanced ALK-rearranged NSCLC, and provide a chance for most of them to reach previously unexpected survivals, transforming this subtype of advanced lung cancer into a chronic disease. However, the development of above mentioned next-generation ALK inhibitors is still in preliminary phases and data are still immature for strong conclusions, thus we will not focus further on these inhibitors but we will discuss more extensively some issues from the use of already approved ALK TKIs in clinical practice.
Special issues of ALK inhibitors
Mechanisms of resistance
The frontline response rates to crizotinib did not exceed 60% and as described by the PROFILE 1014 and 1007 trials, a proportion of patients between 5–15% at the first assessment had stable and progressive disease, These findings suggest an underlying primary resistance (30,31). Regarding the de novo resistance, preclinical evidence showed different crizotinib sensitivity to the different types of ALK variants (variant 1: E13;A20, presenting in 33% of cases, variant 2: E20;A20, presenting in 10%, variant 3a/b: E6a/b;A20, presenting in 29%), with variant 2 much more sensitive to ALK inhibition (60,61). The observed primary resistance and heterogeneous treatment response could also be affected by the false-positive genotyping due to the various techniques used to detect ALK rearrangements (62).
Several mechanisms of acquired resistance to ALK TKIs have been identified through sequencing analysis of pre- and post-progression biopsy specimens as well as in cellular and xenograft models (6,63-67). The major mechanism of resistance to ALK TKIs involves the emergence of secondary on-target mutations. These secondary mutations are recognized in the 20–40% of patients progressing to crizotinib. The gatekeeper mutation of L1196M and the G1269A mutation are the most frequently detected in crizotinib-resistant patients, and they act reducing the affinity of TKI binding in the ATP-pocket (64,68). However, a broad spectrum of additional secondary resistance mutations have been also identified including those that affect the N-terminus of the αC helix (C1156Y, L1152R, and I1151Tins) or the C-terminus of the αC helix (F1174C/L/V); mutations that distort the αC helix to interfere with TKI binding (I1171T/N/S) and solvent-front mutations (D1203N, S1206Y/C, G1202R, G1202del) (64-66,68,69). Each of the four approved ALK TKIs, crizotinib, ceritinib, alectinib and brigatinib, due to their different structures are associated with a specific profile of ALK resistance mutations. For instance, cell lines that carry the I1171 mutations are resistant to alectinib but sensitive to ceritinib, and those that carry the F1174 mutations are resistant to ceritinib but sensitive to alectinib (43,69,70). Due to tumour adaptation after sequential treatment with ALK TKIs, complex resistant mutations such as the C1156Y/I1171N or predominant resistance mutation such as the G1202R are developed to overcome the drug pressure (43). G1202R mutants of ALK generate high-grade resistance to alectinib, brigatinib and ceritinib, but this mutation can be targeted by lorlatinib and other under development ALK TKIs. Moreover, ALK amplification (in 15% of cases) (71) and bypass track activation (in 30% of cases) involving NSCLC drivers such as EGFR, c-KIT and KRAS (65,66,68) have been reported. Other less common mechanisms of resistance to ALK inhibition are the amplification of the fusion ALK gene, reported in the 15% of patients treated mainly with next generation ALK TKIs (43,66,68,71) and the activation of ALK-independent bypass signaling tracks such as EGFR activation, overexpression of neuregulin (NRG1), the ligand for ERBB3 (HER3) and ERBB4 (HER4), MET amplification, PIK3CA mutations, KIT amplification and IGF1R activation reported in the 30–50% of cases resistant to second-generation ALK TKIs. Epithelial to mesenchymal transition (EMT) (72), transformation to SCLC (73,74) and P-gp overexpression (44,75,76) were identified as additional mechanisms of resistance to ALK inhibition. The transformation of ALK-positive NSCLC to SCLC could suggest the presence of a heterogeneous disease, presenting from the beginning and developing under the selected pressure of ALK inhibition. Notably, there are also few reports of cases with SCLC harboring EML4-ALK fusion gene (77,78). P-gp overexpression limits the penetration of crizotinib and ceritinib to CNS, while alectinib, which is not a P-gp substrate, can achieve higher CNS levels (44,75,76). Despite the advances in the molecular basis of resistance to ALK inhibitors, many mechanisms remain still unknown and their identification represents a true challenge in this field of cancer research. For example, in ASCEND-1 study, after re-biopsy before treatment with ceritinib, a secondary ALK mutation (n=5) or ALK gene amplification (n=2) were detected only in a small fraction of 19 crizotinib-resistant patients, while the majority (n=12) maintained the initial ALK translocation. Moreover, in the subgroup analysis of ALTA study presented at ASCO 2016, the ORR was high (>60%) both in patients with or without secondary ALK mutations at baseline, demonstrating an independent activity of brigatinib to ALK mutational status conditioning resistance to crizotinib. On the other hand, responses to brigatinib were also observed despite the baseline presence of two specific mutations (F1174L and G1202R) usually developed after long-term treatment.
Taking together these findings for resistance, we should pinpoint some important notices: (I) the natural ability of NSCLC to develop resistance even to the most potent novel ALK-directed agents; (II) the high clinical value of serial tumor re-biopsy at every recurrence on ALK TKIs; and (III) the promising perspectives of combinatorial treatments of ALK inhibitors with MEK inhibitors, EGFR-directed treatment or immunotherapies.
Behavior of ALK (+) NSCLC with brain metastases in ALK-directed treatments—CNS penetration and activity of ALK inhibitors
Early on, patients with ALK-rearranged NSCLC were observed to be at high risk of developing CNS disease, as observed in ~30% of cases at the time of tumor diagnosis (79) and in 50–60% of patients during crizotinib treatment (80). Crizotinib seems to be weakly active in brain metastases, especially for patients who had previously undergone brain radiotherapy. In a retrospective analysis of the PROFILE 1005 and 1007 trials (80), the IC-ORR and 12-week intracranial disease control rate (IC-DCR) in 18% and 56% of patients, respectively. The median time to CNS progression (IC-TTP) was 7 months in patients with previously untreated brain metastasis, but results were better for patients previously treated with brain radiotherapy who achieved an IC-ORR of 33%, a 12-week IC-DCR of 62% and a median IC-TTP of 13.2 months (80). The PROFILE 1014 trial enrolled 79 patients with baseline previously treated brain metastases and achieved median IC-TTP of 15.7 months in the crizotinib group vs. 12.5 months in the chemotherapy group (81). The 12-week IC-DCR was significantly higher with crizotinib vs. chemotherapy (85% vs. 45%). However, because all the patients had previously received whole brain radiation, the intracranial activity of crizotinib may have been overestimated. The main reason for crizotinib failure in brain disease is its poor blood-brain barrier penetration and subsequently its low CSF/plasma ratios (82,83). Initially for the treatment of CNS disease, many approaches have been tested, including high-dose crizotinib alone (84) or in combination with high-dose pemetrexed (85) and concurrent radiotherapy with continuation of crizotinib (86). In patients who received brain RT without stopping crizotinib, the observed survival benefits suggest that concurrent treatment might represent a successful therapeutic strategy in ALK-rearranged NSCLC with BM (87).
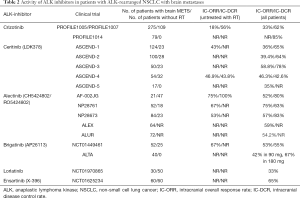
Second and third generation ALK inhibitors penetrate more efficiently penetrate the brain barrier, reaching high concentrations in CSF. Table 2 summarizes the reported CNS activity of ALK inhibitors in patients with brain disease among the abovementioned trials. In ASCEND-1 trial, ceritinib showed an IC-DCR of 80% and 65% in crizotinib-naïve and crizotinib-resistant patients, respectively (36). No differences found between patients received RT or not while the median time to intracranial response was 6 weeks. In crizotinib-naïve patients with measurable BM, IC-ORR was 63% and in crizotinib-resistant patients, 35% (36). In confirmation with ASCEND-1, the ASCEND-2 trial presented an ORR of 40% and a DCR of 85% in 33 crizotinib-resistant patients with measurable brain disease (37) and while the ASCEND-3 trial showed an ORR of 60% and a DCR of 80% in 17 Crizotinib-naïve patients. Synthesizing the results of these studies (ASCEND-1, -2 and -3 trials), a pooled analysis of crizotinib-naïve patients with measurable BM treated with ceritinib reported an IC-ORR of 60%, an IC-DCR of 76% and a median duration of intracranial response 8 months. Another phase II study (ASCEND-7) is currently recruiting participants to confirm the efficacy of ceritinib in patients with ALK (+) NSCLC metastatic to the brain and/or to meninges (NCT02336451). Since the phase II trials (NP28761 and NP28763), alectinib showed to be highly effective against brain metastases with IC-ORR ranging between 52% and 57%. The recent announced data at the ESMO 2017 meeting in Madrid from two separate phase III trials, the ALUR trial and the secondary analysis of the ALEX trial highlighted further the role of alectinib in the control of CNS progression of ALK (+) NSCLC, both in the first-line as well as the second-line treatment setting. The ALUR trial randomized to second-line therapy with either standard relapse chemotherapy or alectinib, 107 patients whose disease had progressed after a previous first-line combination treatment of both platinum-based chemotherapy and crizotinib. Median PFS was significantly longer in the alectinib group compared to the chemotherapy group (9.6 vs. 1.4 months; P<0.001), with a marked difference in CNS response: among patients who had measurable CNS disease at baseline, IC-ORR was 54.2% in those treated with alectinib compared to 0% in the chemotherapy group (P<0.001). As expected, the safety profile of alectinib was more favorable compared to chemotherapy, despite the substantially longer duration of treatment for patients on alectinib (20 versus 6 weeks with chemotherapy). The new subgroup analysis of ALEX trial presented at the meeting, focused specifically on 122 patients who had CNS metastases at baseline and showed that alectinib controls existing CNS metastases and inhibits the formation of new metastases better than crizotinib. This superiority against CNS disease clearly contributes to the overall efficacy of alectinib, limiting the complications from brain metastases themselves as well as from other local treatments such as WBRT. Based on the preliminary results of a phase II trial announced as an abstract at ASCO meeting 2015, brigatinib showed an IC-ORR of 50% in patients with measurable lesions. The CNS activity of lorlatinib is deriving only from a recent not published yet clinical trial that presented a DCR of 65% in patients treated with at least one previous ALK inhibitor. Finally, entrectinib showed high CNS penetration and durable responses both in primary brain tumors and in metastatic brain lesions, and in the phase I trial of ensartinib the IC-ORR was approximately 65%, both in crizotinib-naïve and -resistant patients (Table 2).
Full table
Safety of ALK-TKI inhibitors in ALK (+) NSCLC
The most common grade 1/2 toxicities related to ALK-TKI inhibitors are presented to Table 1, as reported by the different clinical trials. Crizotinib is a well-tolerated inhibitor, associated with an improvement in quality of life and in disease-related symptoms compared to standard chemotherapy. In the PROFILE 1007 and 1014 trials, the discontinuation rate due to toxicity was low (6% and 12%, respectively). The most common grade 3/4 toxicity was hepatotoxicity (15% of patients in both studies). An interesting event, reported during treatment with crizotinib was the reduction of testosterone level in a large sample of male patients with subsequent androgen deficiency and onset of related symptoms such as depression, fatigue, sexual dysfunction, which improved after testosterone supplementation (88). A potential etiology could be the high expression of MET and ALK in testicular tissues and a close monitoring of testosterone levels is recommended. Ceritinib at the starting dose of 750 mg daily led to gastrointestinal AEs in almost all patients (approximately 70–80% of patients had diarrhea, more than 60% nausea, or vomiting, and about 40% presented increased of ALT and AST concentrations in the serum) and 80% of cases required dose adjustment or interruption. The part 1 of a recent multicenter, randomized, open-label, phase 1, study, ASCEND-8 examined whether administering ceritinib, 450 or 600 mg, with a low-fat meal may enhance gastrointestinal tolerability versus 750 mg fasted in patients with ALK-positive NSCLC (either treatment naive or pretreated with chemotherapy and/or crizotinib) (89). In both circumstances ceritinib maintained similar exposure with comparable pharmacokinetic profile while the dose of 450 mg with food presented lower proportion of grade 1 gastrointestinal events (diarrhea: 43.2%, nausea: 29.5% and vomiting: 18.2%), no grade 3–4 events, and no study drug discontinuations, compared to the standard dose (89). Alectinib as a novel ALK TKI presents a sufficiently safe profile with the majority of AEs reported being grades 1 and 2. The most common grade 3–4 AEs included increases of blood creatinine phosphokinase, ALT, and AST. Dose interruption has been reported in 36% of patients and dose reduction in 16%. The overall percentage of serious AEs was 15%. Only 2% of patients discontinued the treatment due to AEs.
A recent pooled safety analysis of ALK TKI inhibitors including ceritinib, crizotinib and alectinib summarized their safety profile (90). The frequencies of therapy-related deaths was no more than 1% (0.6%, 1.0%, and 0.9%, respectively) and the frequencies of AEs leading to treatment withdrawal were 6.9%, 4.5%, and 5.8%, respectively. Among the minor recorded side effects, the most interesting finding was that the long-term exposure to ceritinib was associated with increased frequencies of ≥3 grade hepatotoxicity, fatigue, vomiting, diarrhea and nausea compared to crizotinib or alectinib. For instance, the frequencies of grade ≥3 hepatotoxicity were 22.5%, 7.9% or 3.3%, respectively. In these patients, discontinuation and dose modifications reverse the occurrence of AEs. These toxicities may be developed due to the specific inhibition of IGF1 and insulin receptors by ceritinib and neither by crizotinib nor by alectinib (35). No differences were detected found in the ≥3 grade AEs between first-line and second-line use of crizotinib as well as with ceritinib. Severe neutropenia grade ≥3 occurs significantly more often among patients treated with crizotinib than those treated with ceritinib or alectinib with inhibitory action against the c-Met receptor (90).
Sequencing of ALK inhibitors
The optimal frontline option and the sequence of TKIs are under continuous consideration in the rapidly evolving landscape of ALK inhibitors. On May 2017, FDA broadened the indication of ceritinib to previously untreated ALK-positive metastatic NSCLC and therefore crizotinib is not the only approved agent for the frontline management. In the near future, alectinib will have 1st-line approval on the basis of findings from ALEX and JALEX studies (49,50). By 2018, more ALK inhibitors, such as brigatinib [the ALK in Lung Cancer Trial of Brigatinib in 1st Line (NCT02737501)], lorlatinib (NCT03052608), and ensartinib [the eXalt3 trial (NCT02767804)] are expecting to be available in patients who have not previously received ALK TKIs (91). Among the multiple options for frontline approach of ALK (+) NSCLC, the final choice of 1st line drug for ALK inhibition will be dependent on drug approval patterns, toxicity apprehensions, and cost concerns of individual health-care systems. Before any subsequent therapy, a re-biopsy or liquid biopsy is recommended to analyze molecularly the progressing tumor. Thus, the selection of second ALK inhibitor as the next therapeutic agent should be determined by the recognition of specific ALK mutation that caused the resistance to 1st ALK inhibitor and will be sensitive to the second ALK-directed option (92). Ceritinib and alectinib have been currently approved for second-line setting, as they have demonstrated clinical benefit compared to standard chemotherapy. The identification of an alternative activated pathway plus to ALK inhibition may further help by adding another targeted agent or choosing an ALK-inhibitor with EGFR co-activity (93). In patients who have developed progression after two ALK inhibitors, lorlatinib when becomes available in the market should be also a consideration, especially if a sensitive ALK mutation is identified in the patient’s tumor. For patients who have received two prior ALK inhibitors, a platinum/pemetrexed doublet remains another option. In cases with high PD-L1 expression, immunotherapy could be considered before chemotherapy. Otherwise, patients may undergo systemic chemotherapy or treatment with PD-1 directed agents if the tumor has high PD-L1 expression (92,93).
Conclusions
The identification of a rare but specific molecularly defined subset of NSCLC patients, harboring ALK rearrangements and the approvals of crizotinib, alectinib, ceritinib, and brigatinib have displaced conventional chemotherapy to later setting of evidence-based management of advanced ALK (+) NSCLC, changing completely the every-day practice in lung cancer. However, many challenges remain to be addressed by ongoing trials, including the optimal sequence of ALK inhibitors, the control of CNS disease, the space (if any) for combinatorial treatments and the incorporation of immunotherapies in ALK (+) patients, in order to improve further the personalized approach for this subset of NSCLC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Caccese M, Ferrara R, Pilotto S, et al. Current and developing therapies for the treatment of non-small cell lung cancer with ALK abnormalities: update and perspectives for clinical practice. Expert Opin Pharmacother 2016;17:2253-66. [Crossref] [PubMed]
- Passaro A, Lazzari C, Karachaliou N, et al. Personalized treatment in advanced ALK-positive non-small cell lung cancer: from bench to clinical practice. Onco Targets Ther 2016;9:6361-76. [Crossref] [PubMed]
- Sullivan I, Planchard D. ALK inhibitors in non-small cell lung cancer: the latest evidence and developments. Ther Adv Med Oncol 2016;8:32-47. [Crossref] [PubMed]
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703. [Crossref] [PubMed]
- Markham A. Brigatinib: First Global Approval. Drugs 2017;77:1131-5. [Crossref] [PubMed]
- Karachaliou N, Santarpia M, Gonzalez Cao M, et al. Anaplastic lymphoma kinase inhibitors in phase I and phase II clinical trials for non-small cell lung cancer. Expert Opin Investig Drugs 2017;26:713-22. [Crossref] [PubMed]
- Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008;14:4275-83. [Crossref] [PubMed]
- Li Y, Ye X, Liu J, et al. Evaluation of EML4-ALK fusion proteins in non-small cell lung cancer using small molecule inhibitors. Neoplasia 2011;13:1-11. [Crossref] [PubMed]
- Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 1994;263:1281-4. [Crossref] [PubMed]
- Stoica GE, Kuo A, Aigner A, et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem 2001;276:16772-9. [Crossref] [PubMed]
- Stoica GE, Kuo A, Powers C, et al. Midkine binds to anaplastic lymphoma kinase (ALK) and acts as a growth factor for different cell types. J Biol Chem 2002;277:35990-8. [Crossref] [PubMed]
- Mino-Kenudson M, Chirieac LR, Law K, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res 2010;16:1561-71. [Crossref] [PubMed]
- Iwahara T, Fujimoto J, Wen D, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene 1997;14:439-49. [Crossref] [PubMed]
- George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature 2008;455:975-8. [Crossref] [PubMed]
- Choi YL, Takeuchi K, Soda M, et al. Identification of novel isoforms of the EML4-ALK transforming gene in non-small cell lung cancer. Cancer Res 2008;68:4971-6. [Crossref] [PubMed]
- Takeuchi K, Choi YL, Soda M, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res 2008;14:6618-24. [Crossref] [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [Crossref] [PubMed]
- Chiarle R, Voena C, Ambrogio C, et al. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat Rev Cancer 2008;8:11-23. [Crossref] [PubMed]
- Butrynski JE, D’Adamo DR, Hornick JL, et al. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N Engl J Med 2010;363:1727-33. [Crossref] [PubMed]
- Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 2008;455:971-4. [Crossref] [PubMed]
- Lin E, Li L, Guan Y, et al. Exon array profiling detects EML4-ALK fusion in breast, colorectal, and non-small cell lung cancers. Mol Cancer Res 2009;7:1466-76. [Crossref] [PubMed]
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008;3:13-7. [Crossref] [PubMed]
- Soda M, Isobe K, Inoue A, et al. A prospective PCR-based screening for the EML4-ALK oncogene in non-small cell lung cancer. Clin Cancer Res 2012;18:5682-9. [Crossref] [PubMed]
- Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. [Crossref] [PubMed]
- Kerr KM, Lopez-Rios F. Precision medicine in NSCLC and pathology: how does ALK fit in the pathway? Ann Oncol 2016;27 Suppl 3:iii16-iii24. [Crossref] [PubMed]
- Heydt C, Kostenko A, Merkelbach-Bruse S, et al. ALK evaluation in the world of multiplex testing: Network Genomic Medicine (NGM): the Cologne model for implementing personalised oncology. Ann Oncol 2016;27 Suppl 3:iii25-iii34. [Crossref] [PubMed]
- Cui JJ, Tran-Dube M, Shen H, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 2011;54:6342-63. [Crossref] [PubMed]
- Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012;30:863-70. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [Crossref] [PubMed]
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med 2014;371:2167-77. [Crossref] [PubMed]
- Ou SH, Janne PA, Bartlett CH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol 2014;25:415-22. [Crossref] [PubMed]
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov 2014;4:662-73. [Crossref] [PubMed]
- Marsilje TH, Pei W, Chen B, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem 2013;56:5675-90. [Crossref] [PubMed]
- Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014;370:1189-97. [Crossref] [PubMed]
- Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016;17:452-63. [Crossref] [PubMed]
- Crino L, Ahn MJ, De Marinis F, et al. Multicenter Phase II Study of Whole-Body and Intracranial Activity With Ceritinib in Patients With ALK-Rearranged Non-Small-Cell Lung Cancer Previously Treated With Chemotherapy and Crizotinib: Results From ASCEND-2. J Clin Oncol 2016;34:2866-73. [Crossref] [PubMed]
- Gainor JF, Tan DS, De Pas T, et al. Progression-Free and Overall Survival in ALK-Positive NSCLC Patients Treated with Sequential Crizotinib and Ceritinib. Clin Cancer Res 2015;21:2745-52. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Shaw AT, Kim TM, Crino L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemotherapy and crizotinib (ASCEND-5): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2017;18:874-86. [Crossref] [PubMed]
- Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell 2011;19:679-90. [Crossref] [PubMed]
- Kodama T, Tsukaguchi T, Yoshida M, et al. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett 2014;351:215-21. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in ALK-Rearranged Lung Cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014;74:1023-8. [Crossref] [PubMed]
- Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol 2014;15:1119-28. [Crossref] [PubMed]
- Ou SH, Ahn JS, De Petris L, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol 2016;34:661-8. [Crossref] [PubMed]
- Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1-2 study. Lancet Oncol 2013;14:590-8. [Crossref] [PubMed]
- Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234-42. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Huang WS, Liu S, Zou D, et al. Discovery of Brigatinib (AP26113), a Phosphine Oxide-Containing, Potent, Orally Active Inhibitor of Anaplastic Lymphoma Kinase. J Med Chem 2016;59:4948-64. [Crossref] [PubMed]
- Zhang S, Anjum R, Squillace R, et al. The Potent ALK Inhibitor Brigatinib (AP26113) Overcomes Mechanisms of Resistance to First- and Second-Generation ALK Inhibitors in Preclinical Models. Clin Cancer Res 2016;22:5527-38. [Crossref] [PubMed]
- Rosell R, Gettinger SN, Bazhenova LA, et al. 1330: Brigatinib efficacy and safety in patients (Pts) with anaplastic lymphoma kinase (ALK)-positive (ALK+) non-small cell lung cancer (NSCLC) in a phase 1/2 trial. J Thorac Oncol 2016;11:S114. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem 2014;57:4720-44. [Crossref] [PubMed]
- Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell 2015;28:70-81. [Crossref] [PubMed]
- Bauer TM, Shaw AT, Solomon B, et al. Phase I/II study of PF-06463922, an ALK/ROS1 tyrosine kinase inhibitor, in patients with advanced non-small-cell lung cancer harboring specific molecular alterations. J Clin Oncol 2015;33:abstr TPS2620.
- Shaw AT, Friboulet L, Leshchiner I, et al. Resensitization to Crizotinib by the Lorlatinib ALK Resistance Mutation L1198F. N Engl J Med 2016;374:54-61. [Crossref] [PubMed]
- Farago AF, Le LP, Zheng Z, et al. Durable Clinical Response to Entrectinib in NTRK1-Rearranged Non-Small Cell Lung Cancer. J Thorac Oncol 2015;10:1670-4. [Crossref] [PubMed]
- Sasaki T, Rodig SJ, Chirieac LR, et al. The biology and treatment of EML4-ALK non-small cell lung cancer. Eur J Cancer 2010;46:1773-80. [Crossref] [PubMed]
- Horn L, Pao W. EML4-ALK: honing in on a new target in non-small-cell lung cancer. J Clin Oncol 2009;27:4232-5. [Crossref] [PubMed]
- Heuckmann JM, Balke-Want H, Malchers F, et al. Differential protein stability and ALK inhibitor sensitivity of EML4-ALK fusion variants. Clin Cancer Res 2012;18:4682-90. [Crossref] [PubMed]
- Garraway LA, Janne PA. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov 2012;2:214-26. [Crossref] [PubMed]
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9. [Crossref] [PubMed]
- Sasaki T, Koivunen J, Ogino A, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res 2011;71:6051-60. [Crossref] [PubMed]
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17. [Crossref] [PubMed]
- Crystal AS, Shaw AT, Sequist LV, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science 2014;346:1480-6. [Crossref] [PubMed]
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82. [Crossref] [PubMed]
- Ignatius Ou SH, Azada M, Hsiang DJ, et al. Next-generation sequencing reveals a Novel NSCLC ALK F1174V mutation and confirms ALK G1202R mutation confers high-level resistance to alectinib (CH5424802/RO5424802) in ALK-rearranged NSCLC patients who progressed on crizotinib. J Thorac Oncol 2014;9:549-53. [Crossref] [PubMed]
- Toyokawa G, Hirai F, Inamasu E, et al. Secondary mutations at I1171 in the ALK gene confer resistance to both Crizotinib and Alectinib. J Thorac Oncol 2014;9:e86-7. [Crossref] [PubMed]
- Katayama R, Khan TM, Benes C, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A 2011;108:7535-40. [Crossref] [PubMed]
- Lin JJ, Riely GJ, Shaw AT. Targeting ALK: Precision Medicine Takes on Drug Resistance. Cancer Discov 2017;7:137-55. [Crossref] [PubMed]
- Fujita S, Masago K, Katakami N, et al. Transformation to SCLC after Treatment with the ALK Inhibitor Alectinib. J Thorac Oncol 2016;11:e67-72. [Crossref] [PubMed]
- Oser MG, Niederst MJ, Sequist LV, et al. Transformation from non-small-cell lung cancer to small-cell lung cancer: molecular drivers and cells of origin. Lancet Oncol 2015;16:e165-72. [Crossref] [PubMed]
- Katayama R, Sakashita T, Yanagitani N, et al. P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer. EBioMedicine 2015;3:54-66. [Crossref] [PubMed]
- Tang SC, Nguyen LN, Sparidans RW, et al. Increased oral availability and brain accumulation of the ALK inhibitor crizotinib by coadministration of the P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) inhibitor elacridar. Int J Cancer 2014;134:1484-94. [Crossref] [PubMed]
- Toyokawa G, Takenoyama M, Taguchi K, et al. An extremely rare case of small-cell lung cancer harboring variant 2 of the EML4-ALK fusion gene. Lung Cancer 2013;81:487-90. [Crossref] [PubMed]
- Toyokawa G, Taguchi K, Ohba T, et al. First case of combined small-cell lung cancer with adenocarcinoma harboring EML4-ALK fusion and an exon 19 EGFR mutation in each histological component. J Thorac Oncol 2012;7:e39-41. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Costa DB, Shaw AT, Ou SH, et al. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol 2015;33:1881-8. [Crossref] [PubMed]
- Solomon BJ, Cappuzzo F, Felip E, et al. Intracranial Efficacy of Crizotinib Versus Chemotherapy in Patients With Advanced ALK-Positive Non-Small-Cell Lung Cancer: Results From PROFILE 1014. J Clin Oncol 2016;34:2858-65. [Crossref] [PubMed]
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. [Crossref] [PubMed]
- Metro G, Lunardi G, Floridi P, et al. CSF Concentration of Crizotinib in Two ALK-Positive Non-Small-Cell Lung Cancer Patients with CNS Metastases Deriving Clinical Benefit from Treatment. J Thorac Oncol 2015;10:e26-7. [Crossref] [PubMed]
- Kim YH, Ozasa H, Nagai H, et al. High-dose crizotinib for brain metastases refractory to standard-dose crizotinib. J Thorac Oncol 2013;8:e85-6. [Crossref] [PubMed]
- Gandhi L, Drappatz J, Ramaiya NH, et al. High-dose pemetrexed in combination with high-dose crizotinib for the treatment of refractory CNS metastases in ALK-rearranged non-small-cell lung cancer. J Thorac Oncol 2013;8:e3-5. [Crossref] [PubMed]
- Takeda M, Okamoto I, Nakagawa K. Clinical impact of continued crizotinib administration after isolated central nervous system progression in patients with lung cancer positive for ALK rearrangement. J Thorac Oncol 2013;8:654-7. [Crossref] [PubMed]
- Johung KL, Yeh N, Desai NB, et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J Clin Oncol 2016;34:123-9. [Crossref] [PubMed]
- Weickhardt AJ, Doebele RC, Purcell WT, et al. Symptomatic reduction in free testosterone levels secondary to crizotinib use in male cancer patients. Cancer 2013;119:2383-90. [Crossref] [PubMed]
- Cho BC, Kim DW, Bearz A, et al. ASCEND-8: A Randomized Phase 1 Study of Ceritinib, 450 mg or 600 mg, Taken with a Low-Fat Meal versus 750 mg in Fasted State in Patients with Anaplastic Lymphoma Kinase (ALK)-Rearranged Metastatic Non-Small Cell Lung Cancer (NSCLC). J Thorac Oncol 2017;12:1357-67. [Crossref] [PubMed]
- Zhu Q, Hu H, Weng DS, et al. Pooled safety analyses of ALK-TKI inhibitor in ALK-positive NSCLC. BMC Cancer 2017;17:412. [Crossref] [PubMed]
- Costa DB. Ascending role of next-generation ALK inhibitors. Lancet Oncol 2017;18:837-9. [Crossref] [PubMed]
- Gadgeel SM. Sequencing of ALK Inhibitors in ALK+ Non-Small Cell Lung Cancer. Curr Treat Options Oncol 2017;18:36. [Crossref] [PubMed]
- Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA 2014;311:1998-2006. [Crossref] [PubMed]


