Peri-procedural antibiotic prophylaxis in ventricular septal defect: a case study to re-visit guidelines
Introduction
The current American Heart Association (AHA)/American College of Cardiology (ACC) recommendations for infective endocarditis (IE) antibiotic prophylaxis have precluded the need for antibiotics in patients with acyanotic congenital hemodynamically insignificant ventricular septal defects (VSDs) (1). The rationale for updated guidelines was absence of data to support the use of prophylactic antibiotics in patients with VSD. We present a case of a young female with small congenital VSD who did not receive prophylactic antibiotics and developed bivalvular endocarditis after vaginal delivery.
Case presentation
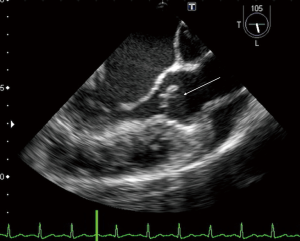
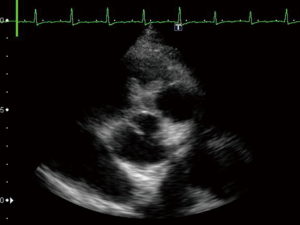
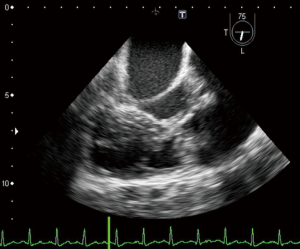
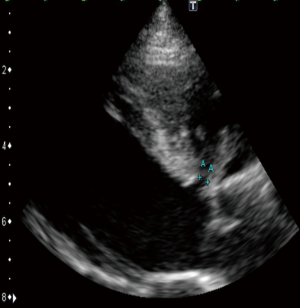
A 23-year-old woman with congenital small-uncomplicated VSD presented to the ED thirty-seven days post-partum complaining of fever, chills and fatigue. She appeared ill with tachycardia, a grade III/IV harsh diastolic murmur in the pulmonary area and decreased bibasilar lung sounds. Blood cultures were positive for streptococcus viridans. Transthoracic echocardiogram (TTE) and transesophageal echocardiogram (TEE) showed a large vegetation on the pulmonic valve (PV) with severe pulmonary insufficiency (Figures 1,2), a small to medium sized vegetation on the aortic valve (AV) with moderate aortic insufficiency (Figure 3) and a 3 mm small restrictive peri-membranous VSD (Figure 4). TEE showed that the VSD color flow jet was directed at the PV (Figure 5). Bacteremia resolved with prolonged antibiotics therapy. However she developed new congestive heart failure (CHF) with left ventricular (LV) systolic dysfunction. The LV ejection fraction was noticed to be 30% as compared to normal function at presentation. The decision was made to close the VSD, replace the PV and the AV. VSD was closed, however due to the relatively small size of the pulmonary artery and the aortic root, PV and AV replacement was not feasible. Our patient’s CHF responded well to medical therapy and she remains symptom-free on long term follow up. Two years later, TTE done showed complete healing of the PV/AV (Figure 6).
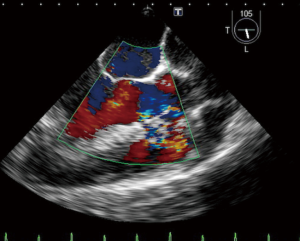
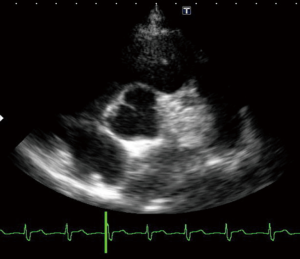
Discussion
VSD is the most common congenital heart disease. Most of the small VSD’s spontaneously close and require no intervention (2). Small VSD’s are hemodynamically non-significant. However, they are associated with high velocity jet from the left ventricle to right ventricle. In peri-membranous VSD, the high velocity jet can be directed towards the right ventricular outflow tract (RVOT) and PV, potentially damaging the endocardial lining of the PV, pre-disposing it to IE. In fact, unrepaired VSD is the most frequent congenital heart disease associated with risk of IE (3). Previous studies have reported risk of IE to be 1.5% to 2.7% per 1,000 patients in patients with unrepaired VSD (4,5). The incidence of IE was as high as 14.5/1,000 patients in second natural history study (6). As seen in our case, IE in VSD can involve multiple valves (7). Surgical repair of the VSD reduces the risk of IE by fifty percent (4).
We believe that presence of unrepaired small VSD was the predisposing factor for development of IE after vaginal delivery in our patient. To our knowledge, the patient did not receive any prophylactic antibiotics for IE prior to vaginal delivery. The PV had a very large vegetation with complete distortion of the valve anatomy and severe regurgitation. The AV had a small to medium sized vegetation with moderate regurgitation. This patient had a small peri-membranous VSD (3 mm) causing left to right shunt with high velocity (5 m/sec), that was directed at the RVOT and PV, which was the likely predisposing factor for denudation and seeding of PV with streptococcal bacteria. The mechanism of AV involvement is less clear; however, it is possible that turbulent jet in the LV outflow tract caused by VSD led to endocardial damage and IE of the AV. The current ACC/AHA guidelines do not necessitate IE prophylaxis in low to moderate risk individuals, which include non-cyanotic congenital heart diseases (e.g., VSD), due to lack of evidence that prophylactic antibiotics offer any benefit. The rationale for limiting IE prophylaxis to high risk patients include: (I) very little benefit of antibiotic prophylaxis in IE prevention due to low incidence of IE in general public; (II) bacteremia is more likely after routine oral hygiene such as tooth brushing rather than oral or genitourinary or gastrointestinal tract instrumentation; (III) potential side effects of antibiotics. These recommendations are probably appropriate in patients with low risk congenital heart disease (6). The annualized risk of IE for unrepaired VSD is considered high (3.8/1,000 cases per 1,000 patient-years) (6). There are no randomized data on efficacy of antibiotic prophylaxis for IE in VSD cases after normal vaginal delivery. Di Filippo pointed out that congenital heart disease (CHD) lesions (such as VSD) with high velocity and turbulent flow are not considered as predisposing risk factors for IE in the current guidelines. However, patients with VSD are at higher risk of IE among all congenital heart disease patients (8). We believe that ACC/AHA guidelines exclude unrepaired VSD patients from antibiotic prophylaxis, who might be at high risk of IE. Unrepaired VSD carries a high lifetime risk of IE among all congenital heart diseases. As highlighted in our case, a small hemodynamically nonsignificant peri-membranous VSD can indeed increase the risk of IE after vaginal delivery. Patients with VSD may be considered for prophylactic antibiotics in the perioperative period.
Incidence of bacteremia after normal vaginal delivery is not known, and there is paucity of data on incidence of postpartum IE with normal vaginal delivery. In a systematic review of 90 patients, congenital heart disease was a risk factor in 12% cases of postpartum IE. Staphylococcus and streptococcus species were the most common pathogens of the postpartum IE (9). Postpartum IE most commonly involves mitral and aortic valves, and is associated with high mortality and morbidity (10). However, this study however did not describe what proportion of patients developed IE after normal vaginal delivery. The current ACC/AHA guidelines do not recommend antibiotic prophylaxis for vaginal or cesarean delivery except for patient with CHD such as unrepaired or palliated cyanotic heart disease, or for surgically constructed palliative shunts or patients with prosthetic heart valves or prior IE (11). We believe that antibiotic prophylaxis can potentially benefit VSD patients in the peripartum period since it is associated with high risk of IE. Single dose of second generation cephalosporin (e.g., cefotetan or cefoxitin) can be considered for antibiotic prophylaxis prior to vaginal delivery for VSD patients. Clindamycin 600 mg IV or erythromycin 500 mg IV can be considered as an alternative for penicillin allergic patients (12).
Conclusions
This case highlights the fact that small VSD’s that are hemodynamically insignificant can predispose to IE. Antibiotic prophylaxis may be considered for VSD defects peri-operatively.
Acknowledgements
None.
Footnote
Conflicts of Interest: This case report was presented at ACC 2016 as a poster.
Informed Consent: Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Nishimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 Guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2008;52:676-85. [Crossref] [PubMed]
- Zhang J, Ko JM, Guileyardo JM, et al. A review of spontaneous closure of ventricular septal defect. Proc (Bayl Univ Med Cent) 2015;28:516-20. [Crossref] [PubMed]
- Li W, Somerville J. Infective endocarditis in the grown-up congenital heart (GUCH) population. Eur Heart J 1998;19:166-73. [Crossref] [PubMed]
- Gersony WM, Hayes CJ, Driscoll DJ, et al. Bacterial endocarditis in patients with aortic stenosis, pulmonary stenosis, or ventricular septal defect. Circulation 1993;87:I121-6. [PubMed]
- Morris CD, Reller MD, Menashe VD. Thirty-year incidence of infective endocarditis after surgery for congenital heart defect. JAMA 1998;279:599-603. [Crossref] [PubMed]
- NICE clinical guidelines 64, Prophylaxis Against Infective Endocarditis: Antimicrobial Prophylaxis Against Infective Endocarditis in Adults and Children Undergoing Interventional Procedures. March 2008. Available online: https://www.nice.org.uk/guidance/cg64
- Birkenkamp KE, Jin JJ, Shivashankar R, et al. Ventricular septal defect and bivalvular endocarditis. Avicenna J Med 2015;5:21-3. [Crossref] [PubMed]
- Di Filippo S. Prophylaxis of infective endocarditis in patients with congenital heart disease in the context of recent modified guidelines. Arch Cardiovasc Dis 2012;105:454-60. [Crossref] [PubMed]
- Pharis CS, Conway J, Warren AE, et al. The impact of 2007 infective endocarditis prophylaxis guidelines on the practice of congenital heart disease specialists. Am Heart J 2011;161:123-9. [Crossref] [PubMed]
- Kebed KY, Bishu K, Al Adham RI, et al. Pregnancy and postpartum infective endocarditis: a systematic review. Mayo Clin Proc 2014;89:1143-52. [Crossref] [PubMed]
- Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 Guidelines for the Management of Adults with Congenital Heart Disease: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to develop guidelines for the management of adults with congenital heart disease). Circulation 2008;118:2395-451. [Crossref] [PubMed]
- van Schalkwyk J, Van Eyk N. Infectious Diseases Committee. Antibiotic prophylaxis in obstetric procedures. J Obstet Gynaecol Can 2010;32:878-84. [Crossref] [PubMed]