Macrolide therapy is associated with reduced mortality in acute respiratory distress syndrome (ARDS) patients
Introduction
Clinical trials in patients with chronic inflammatory lung diseases like diffuse panbronchiolitis (1-4), cystic fibrosis (5-7) and chronic obstructive pulmonary disease (8) show favorable clinical effects of long-term macrolide therapy. Benefit of macrolide therapy in these patients is likely not the consequence of an antimicrobial effect as used dosages are too low to have antibacterial properties, and also because the respiratory pathogens involved in these chronic diseases are usually insensitive to macrolides. In vitro and ex vivo studies show that macrolides attenuate cytokine production by several cell types (9-12), and alter the functioning of polymorphonuclear cells (13-17). Thus, the beneficial effects of macrolide therapy in these diseases could come from its anti-inflammatory rather than from antimicrobial effects (18).
There is mounting evidence for benefit of macrolide therapy in acute inflammatory lung diseases as well. A recently published small trial suggests an association between macrolide therapy and decreased mortality and shorter duration of mechanical ventilation in patients with the acute respiratory distress syndrome (ARDS) (19). This finding is echoed in animal models of lung injury (20-26) in which macrolide therapy reduces neutrophil influx (21), lowers levels of several pro-inflammatory mediators (20,22,23,26), reduces pulmonary edema in the lungs (25), and also diminishes the amount of mucus in the airways (24).
To test the hypothesis whether macrolides affect outcomes of patients with ARDS, we determined the association between low-dose macrolide therapy prescribed for other reasons than infection and mortality in patients with ARDS. Patients were included in a large observational study performed in the intensive care units (ICUs) of two academic hospitals in the Netherlands (27). We focused on the effect of macrolide therapy on mortality in the whole cohort, but also determined and compared the association between macrolide therapy and outcome in patients with pulmonary versus non-pulmonary ARDS, and two biological phenotypes of ARDS based on plasma levels of biomarkers for inflammation, coagulation and vascular injury (28).
Methods
Study design and setting
This is an unplanned secondary analysis of the “Molecular Diagnosis and Risk Stratification of Sepsis” (MARS) study, a project performed in the ICUs of the Academic Medical Center, Amsterdam, the Netherlands and the University Medical Center Utrecht, Utrecht, the Netherlands (27). Both departments are closed-format ICUs where a team of board-certified ICU physicians and fellows, and board-certified ICU nurses care for a mixed medical-surgical patient population. The typical nurse-to-patient ratio in these ICUs is 1:1 to 1:2, depending on disease severity, and standard care for ARDS patients consists of lung-protective mechanical ventilation with low tidal volumes, higher levels of positive end-expiratory pressure, and prone ventilation if necessary. Furthermore, a restrictive fluid strategy is followed, and patients receive analgo-sedation using sedation scales with bolus sedation.
Ethical considerations
The local ethical committee approved the MARS study (UMC Utrecht; number, 10-056C). This committee also approved use of an opt-out consent procedure, in which participants were notified of the study in writing by a brochure provided at ICU admission with an attached opt-out card that could be completed by the patient or by his or her legal representative in case of unwillingness to participate.
Inclusion and exclusion criteria
The parent MARS study included consecutive adult patients admitted to the ICU with an expected length of stay of more than 24 hours for the duration of 3 years (January 2011 to January 2014). The parent study had no other inclusion or exclusion criteria.
The present analysis exclusively included patients meeting ARDS criteria at any time during admission to the ICU who received macrolide therapy for other reasons than (presumed) infection.
ARDS was diagnosed by a dedicated team of researchers who were trained in the appropriate use of the American-European Consensus Criteria (29). We found that 100% patients would have fulfilled the criteria of the Berlin definition for ARDS (30), in which patients were classified as having mild, moderate, or severe ARDS using the first day PaO2/FiO2.
Endpoints
The primary outcome was all-cause 30-day mortality.
Data collected
Causes for ARDS were captured and categorized as “pneumonia”, “aspiration”, “other pulmonary causes” (i.e., inhalation trauma, near drowning), “non-pulmonary sepsis”, “trauma or mayor surgery”, “pancreatitis” and “other non-pulmonary causes” (i.e., blood transfusion, toxic medication).
In the event of multiple causes for ARDS, each cause was scored separately. The Acute Physiology and Chronic Health Evaluation (APACHE) IV score (31) was calculated from physiologic data collected in the first 24 hours after ICU admission.
Data on macrolide therapy data, including type of macrolide prescribed and duration, were captured from the day of ICU admission until ICU discharge. The exact dosage was not recorded; the standard practice for describing erythromycin for prokinetic purposes is twice daily 200 mg intravenously. Macrolides exposure was regarded as any low-dose macrolide administration during ICU admission after ARDS diagnosis, irrespectively of the timing or duration.
Phenotypes
In addition to the crude analysis of the effect of macrolide therapy on mortality in the whole cohort, we determined the association between macrolide therapy and outcome in different phenotypes. First, we chose to distinguish pulmonary from non-pulmonary ARDS. We also divided the whole cohort into two previously described biological phenotypes based on plasma concentrations of 20 biomarkers (28). Biological phenotype I is characterized by lower levels of inflammatory markers in plasma compared to biological phenotype II, which represents a hyperinflammatory state (28). The latter analysis was only possible in a subset of patients for whom plasma biomarker levels were available.
Analysis plan
The primary analysis consisted of a logistic regression analysis to test the association between macrolide therapy and 30-day mortality. In this analysis, the association was adjusted for the APACHE IV score. To confirm the association, we subsequently performed a propensity score matched analysis, matching one patient receiving macrolide treatment with three patients not receiving macrolide treatment. We calculated the propensity score for exposure to macrolides by a multivariable regression with low-dose macrolides as the dependent variable, including factors potentially related to administration of low-dose macrolides as the independent variables. The following factors were included in the propensity score: hospital of admission, APACHE IV score, maximum lactate level, presence of diabetes, presence of chronic cardiovascular or respiratory insufficiency, chronic renal insufficiency, presence of congestive heart failure, chronic obstructive pulmonary disease, body mass index, liver cirrhosis, peptic ulcer disease and treatment for peptic ulcers.
Next, we analyzed patients separately according to the primary cause of ARDS (pulmonary versus extra-pulmonary cause). Within these two groups we followed the exact same steps as described above. Finally, we stratified patients into the two biological phenotype groups, i.e., “type I” and “type II” (28), and repeated the analyses.
Statistical analysis
Baseline characteristics were compared using Student’s t-test or Mann-Whitney U-tests where appropriate. Categorical variables were compared with Chi-square test or Fisher’s exact test. Continuous data are expressed median (interquartile range) and categorical variables as number (percentage). An association with the primary outcome 30-day mortality was sought using logistic regression, and adjusted for APACHE IV. Then logistic regression was repeated with stratification for the phenotypes (pulmonary/non-pulmonary cause or biological phenotypes), with adjustment for confounders and propensity scores.
All analyses were performed in R through the R-studio interface using the latest version of the packages “lrm”, “ggplot2” (The R Foundation for Statistical Computing, Vienna, Austria). A P value <0.05 was used to determine statistical significance for all tests.
Results
Patients
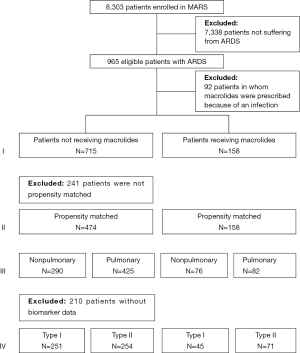
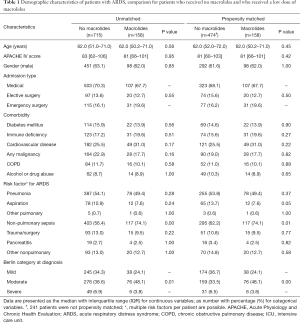
Of the 8,303 patients included in the MARS study, 965 patients were diagnosed with ARDS (Figure 1). Of them, 92 patients were excluded because they had received macrolide therapy because of an infection, which resulted in 873 patients for the primary analysis, of whom 158 patients who had received macrolide therapy. Baseline characteristics and causes for ARDS are presented in Table 1. The most frequent reported causes of ARDS were “pneumonia” and “non-pulmonary sepsis”. In the macrolide therapy group more patients had moderate ARDS and fewer had mild or severe ARDS compared to the no exposure group. Patients who received macrolides more often had non-pulmonary sepsis as risk factor for ARDS.
Full table
Macrolide therapy
The most frequently prescribed macrolide was erythromycin (97%). Other macrolides used were azithromycin and clarithromycin. These macrolides were prescribed for their prokinetic action on the gut. The majority of patients (78%) received macrolide therapy within the first 5 days of admission {median, 2 [1–5] days}, over a median of 3 [1–4] days.
Outcome
Macrolide therapy was associated with a lower 30-day mortality (Table 2). Adjusting for APACHE IV score did not change this association [OR, 0.64 (0.43–0.96), P=0.03]. Macrolide therapy remained significantly associated with a lower 30-day mortality in the propensity score matched cohort (Table 2).

Full table
Clinical and laboratory biological phenotypes
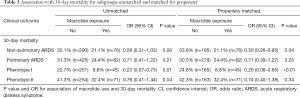
Associations between macrolide therapy and mortality were statistically significant only in patients with non-pulmonary ARDS compared to pulmonary ARDS, and only after propensity score matching (Table 3). Associations between macrolide therapy and mortality were statistically significant only in patients with biological phenotype I, compared to phenotype II for both matched and unmatched analyses (Table 3).
Full table
Discussion
The results of this observational study in patients with ARDS can be summarized as follows: (I) in ARDS patients low-dose macrolide therapy is associated with a lower 30-day mortality; (II) this association was also significant after correcting for APACHE IV score; (III) and remained significant when matched for propensity score; (IV) in subgroup analyses the association between macrolide therapy and mortality was statistically significant in patients with non-pulmonary ARDS and (V) in patients with biological phenotype I.
An important strength of this analysis is that it was performed in a well-described and large cohort of ARDS patients, in which the diagnosis of ARDS was made prospectively by well-trained researchers with extensive experience in using diagnostic criteria for ARDS. We were able to reclassify all patients according to the latest diagnostic criteria, i.e., the Berlin definition for ARDS (30). Another strength is that we restricted the analysis to macrolide therapy for reasons other than infection, thereby excluding the effects of antibacterial properties of macrolides. We enriched the analyses by repeating it in a propensity score matched cohort. Finally, we performed several additional analyses to explore whether benefit exists in pulmonary as well as in non-pulmonary ARDS, and in the different biological phenotypes of ARDS.
The results of the present study are in line with those from previous investigations. In one secondary analysis of a randomized controlled trial in ARDS patients, patients who had received macrolide therapy within 24 hours of trial enrollment had lower 180-day mortality (19). In a retrospective study in patients with sepsis-related ARDS, macrolide therapy given within 24 hours after ARDS onset was associated with lower 60-day mortality (32). Of note, in both investigations macrolide therapy could have been given for infections, while these patients were excluded in the present study. Also, the two previous investigations concerned relatively small patient cohorts.
The exact mechanisms in which macrolides influence outcome in ARDS patients remain largely speculative. Given the numerous reports on the effects of macrolides on cytokine production and neutrophil function, it is an attractive thought to consider this as the main path of action. It should be noted that the benefit of macrolide therapy in other pulmonary diseases, like diffuse panbronchiolitis (1-4), cystic fibrosis (5-7) and chronic obstructive pulmonary disease (8) seem to require long-term treatment (i.e., weeks to months), while patients in the present investigation received macrolides for relative short periods (i.e., days). On the other hand, several animal studies (20-26) as well as in vitro experiments using whole blood stimulations with different stimuli (12,13,33) showed early effects of macrolides.
One remarkable finding is the differential effect of macrolide exposure in biological phenotype I, as compared to biological phenotype II. Biological phenotype I, characterized as the less inflamed type (28), has a greater benefit from macrolides. This seems to be contradictory to the alleged mechanism of action of macrolides and we cannot explain this finding with the currently available data. However, a differential response to certain therapeutic interventions between the two phenotypes has been described before. Patients with a hyperinflammatory phenotype, which shows strong resemblance to phenotype II described in this study (28,34) showed benefit from a higher positive end-expiratory pressure (PEEP) and a conservative fluid strategy (34,35). This is the first study to provide data which suggest a selective advantage of phenotype I to a pharmacological intervention specifically targeting the immune system.
As this is an observational study, we should be careful in interpreting the results. We suggest that these findings can be used for further exploration of the application of macrolides as pharmacological treatment for ARDS and to inform future randomized trials for their selection of patients, e.g., by including patients with non-pulmonary ARDS or biological phenotype I.
There are several limitations to this investigation, including that we cannot exclude the potential influence of other confounders than those used in the analysis of the propensity score matched cohort. It could be that some reasons to prescribe or withhold macrolides, which were not available to us from the MARS database, have a stronger association with mortality than the macrolide use itself. Second, as we chose to exclude patients who received macrolides for an infection, we cannot conclude anything about the effect of the higher dosages of macrolides, or about these patients for which high-dose macrolides had been prescribed. Third, as we performed an observational study we could not control for the timing in which macrolides were given. Even though most patients received macrolides early in the course of ARDS, i.e., within 5 days, it could be that macrolides are more effective when given as quickly as possible after onset, like described before (19,32). Fourth, our conclusions may not be generalizable to patients admitted outside to the two tertiary hospitals in the Netherlands, which collected data for the parent study.
In conclusion, this study suggests low-dose macrolides to be associated with lower hospital mortality in patients with ARDS, and that the effect of macrolides varies by ARDS etiology as well as specific immunologic phenotype.
Acknowledgements
The authors sincerely thank everyone involved in the MARS project. More information on this public, private consortium can be found on http://www.ctmm.nl/nl/projecten/infectie/mars
Funding: The MARS project was funded by the Center for Translational Molecular Medicine, under project number 10.13039/501100006020.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The local ethical committee approved the MARS study (UMC Utrecht; number, 10-056C). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Kadota J, Mukae H, Ishii H, et al. Long--term efficacy and safety of clarithromycin treatment in patients with diffuse panbronchiolitis. Respir Med 2003;97:844-50. [Crossref] [PubMed]
- Yamamoto M, Kondo A, Tamura M, et al. Long--term therapeutic effects of erythromycin and newquinolone antibacterial agents on diffuse panbronchiolitis. Nihon Kyobu Shikkan Gakkai Zasshi 1990;28:1305-13. [PubMed]
- Nagai H, Shishido H, Yoneda R, et al. Long--term low--dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration 1991;58:145-9. [Crossref] [PubMed]
- Kudoh S, Uetake T, Hagiwara K, et al. Clinical effects of low--dose long--term erythromycin chemotherapy on diffuse panbronchiolitis. Nihon Kyobu Shikkan Gakkai Zasshi 1987;25:632-42. [PubMed]
- Wolter J, Seeney S, Bell S, et al. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002;57:212-6. [Crossref] [PubMed]
- Equi A, Balfour-Lynn IM, Bush A, et al. Long term azithromycin in children with cystic fibrosis: a randomised, placebo-controlled crossover trial. Lancet 2002;360:978-84. [Crossref] [PubMed]
- Gaylor AS, Reilly JC. Therapy with macrolides in patients with cystic fibrosis. Pharmacotherapy 2002;22:227-39. [Crossref] [PubMed]
- Ni W, Shao X, Cai X, et al. Prophylactic use of macrolide antibiotics for the prevention of chronic obstructive pulmonary disease exacerbation: a meta-analysis. PLoS One 2015;10:e0121257. [Crossref] [PubMed]
- Takizawa H, Desaki M, Ohtoshi T, et al. Erythromycin suppresses interleukin 6 expression by human bronchial epithelial cells: a potential mechanism of its anti-inflammatory action. Biochem Biophys Res Commun 1995;210:781-6. [Crossref] [PubMed]
- Morikawa K, Watabe H, Araake M, et al. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother 1996;40:1366-70. [PubMed]
- Khair OA, Devalia JL, Abdelaziz MM, et al. Effect of erythromycin on Haemophilus influenzae endotoxin-induced release of IL-6, IL-8 and sICAM-1 by cultured human bronchial epithelial cells. Eur Respir J 1995;8:1451-7. [PubMed]
- Schultz MJ, Speelman P, van der Poll T. Erythromycin inhibits Pseudomonas aeruginosa-induced tumour necrosis factor-alpha production in human whole blood. J Antimicrob Chemother 2001;48:275-8. [Crossref] [PubMed]
- Schultz MJ, Speelman P, Hack CE, et al. Intravenous infusion of erythromycin inhibits CXC chemokine production, but augments neutrophil degranulation in whole blood stimulated with Streptococcus pneumoniae. J Antimicrob Chemother 2000;46:235-40. [Crossref] [PubMed]
- Hodge S, Hodge G, Brozyna S, et al. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur Respir J 2006;28:486-95. [Crossref] [PubMed]
- Labro MT, Abdelghaffar H. Immunomodulation by macrolide antibiotics. J Chemother 2001;13:3-8. [Crossref] [PubMed]
- Anderson R, Fernandes AC, Eftychis HE. Studies on the effects of ingestion of a single 500 mg oral dose of erythromycin stearate on leucocyte motility and transformation and on release in vitro of prostaglandin E2 by stimulated leucocytes. J Antimicrob Chemother 1984;14:41-50. [Crossref] [PubMed]
- Wenisch C, Parschalk B, Zedtwitz-Liebenstein K, et al. Effect of single oral dose of azithromycin, clarithromycin, and roxithromycin on polymorphonuclear leukocyte function assessed ex vivo by flow cytometry. Antimicrob Agents Chemother 1996;40:2039-42. [PubMed]
- Amsden GW. Anti-inflammatory effects of macrolides--an underappreciated benefit in the treatment of community-acquired respiratory tract infections and chronic inflammatory pulmonary conditions? J Antimicrob Chemother 2005;55:10-21. [Crossref] [PubMed]
- Walkey AJ, Wiener RS. Macrolide antibiotics and survival in patients with acute lung injury. Chest 2012;141:1153-9. [Crossref] [PubMed]
- Sato K, Suga M, Akaike T, et al. Therapeutic effect of erythromycin on influenza virus-induced lung injury in mice. Am J Respir Crit Care Med 1998;157:853-7. [Crossref] [PubMed]
- Kawashima M. Inhibitory effects of 14-membered ring macrolide antibiotics on bleomycin-induced acute lung injury. Lung 2002;180:73-89. [Crossref] [PubMed]
- Leiva M, Ruiz-Bravo A, Jimenez-Valera M. Effects of telithromycin in in vitro and in vivo models of lipopolysaccharide-induced airway inflammation. Chest 2008;134:20-9. [Crossref] [PubMed]
- Tamaoki J, Kondo M, Kohri K, et al. Macrolide antibiotics protect against immune complex-induced lung injury in rats: role of nitric oxide from alveolar macrophages. J Immunol 1999;163:2909-15. [PubMed]
- Tamaoki J, Takeyama K, Yamawaki I, et al. Lipopolysaccharide-induced goblet cell hypersecretion in the guinea pig trachea: inhibition by macrolides. Am J Physiol 1997;272:L15-9. [PubMed]
- Tamaoki J, Tagaya E, Yamawaki I, et al. Effect of erythromycin on endotoxin-induced microvascular leakage in the rat trachea and lungs. Am J Respir Crit Care Med 1995;151:1582-8. [Crossref] [PubMed]
- Azuma A, Furuta T, Enomoto T, et al. Preventive effect of erythromycin on experimental bleomycin-induced acute lung injury in rats. Thorax 1998;53:186-9. [Crossref] [PubMed]
- Klein Klouwenberg PM, Ong DS, Bos LD, et al. Interobserver agreement of Centers for Disease Control and Prevention criteria for classifying infections in critically ill patients. Crit Care Med 2013;41:2373-8. [Crossref] [PubMed]
- Bos LD, Schouten LR, van Vught LA, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax 2017;72:876-83. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- Zimmerman JE, Kramer AA, McNair DS, et al. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med 2006;34:1297-310. [Crossref] [PubMed]
- Kawamura K, Ichikado K, Takaki M, et al. Efficacy of azithromycin in sepsis-associated acute respiratory distress syndrome: a retrospective study and propensity score analysis. Springerplus 2016;5:1193. [Crossref] [PubMed]
- Schultz MJ, Speelman P, Zaat S, et al. Erythromycin inhibits tumor necrosis factor alpha and interleukin 6 production induced by heat-killed Streptococcus pneumoniae in whole blood. Antimicrob Agents Chemother 1998;42:1605-9. [PubMed]
- Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2014;2:611-20. [Crossref] [PubMed]
- Famous KR, Delucchi K, Ware LB, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med 2017;195:331-8. [PubMed]