Interaction between peri-operative blood transfusion, tidal volume, airway pressure and postoperative ARDS: an individual patient data meta-analysis
Introduction
Transfusion of blood products is a well-known risk factor for acute respiratory distress syndrome (ARDS) in both critically and non-critically ill patients (1-6). Peri-operative transfusion of blood products is also recognized as a risk factor for pulmonary complications after surgery (7-9). Remarkably, while half of all issued blood products are transfused peri-operatively (7-9), the number of studies investigating the association with postoperative pulmonary complications is low.
Mechanical ventilation using high tidal volumes and/or high airway pressures is associated with worse outcomes in patients with ARDS and probably also in patients who do not meet ARDS criteria at the onset of ventilation (10,11). Numerous studies in surgical patients suggest that even short-term intra-operative ventilation with higher tidal volumes and/or higher airway pressures increased the risk for postoperative pulmonary complications and prolonged duration of ventilation and hospital stay (11,12).
It is conceivable that an interaction between the effects of peri-operative transfusion and higher tidal volumes and/or higher airway pressures during surgery on the development of postoperative ARDS exists. Lung injury following transfusion may occur via a ‘double hit mechanism’, in which the first hit primes pulmonary neutrophils and endothelium (3). Mediators in transfused blood products then could serve as a second hit by a further activation of neutrophils and the endothelial cells, finally resulting in lung capillary permeability and subsequent pulmonary edema (3). Observational studies have shown that mechanical ventilation and high peak airway pressure are risk factors for the development of lung injury following transfusion in critically ill patients (13,14). The interaction between transfusion and ventilation parameters in patients with healthy lungs under anesthesia for surgery has never been assessed.
We hypothesized that peri-operative transfusion is associated with the development of postoperative ARDS, and that this association is dependent on the tidal volume size and/or airway pressure level during intra-operative ventilation of patients under anesthesia for surgery.
Methods
The full methodology of the present meta-analysis is available in the Supplement I. The statistical analysis plan has been published previously (15).
Search strategy
A search strategy followed Medical Subject Headings and Keywords (protective ventilation OR lower tidal volume OR low tidal volume OR positive end-expiratory pressure OR PEEP).
Selection of studies
Observational studies or randomized controlled trials of ‘protective’ intra-operative ventilation using lower tidal volumes identified by the search and reporting outcomes of interest were screened for inclusion. Inclusion criteria were: use of lower versus higher tidal volume and/or use of higher versus lower PEEP in each arm; adults (i.e., age >18 years); and patients receiving ventilation in the setting of general anesthesia for surgery. Investigations were excluded from this meta-analysis if they: (I) included patients with pre-existing ARDS; or (II) reported on patients receiving ventilation in a non-surgical setting (i.e., in the intensive care unit).
Transfusion and ventilation parameters
After approval, corresponding authors of retrieved studies or trials were asked to send information on ventilation parameters obtained hourly during the surgical procedure. In addition to ventilatory parameters, authors were asked to provide the number and types of transfusions during the surgical procedure. A blood transfusion was defined as the infusion of red blood cell (RBC) and/or fresh frozen plasma (FFP) in the perioperative period. The blood products were counted as units since we could not collect data regarding volumes of units.
Primary outcome
The primary outcome was development of postoperative ARDS during follow-up.
Secondary outcomes
Secondary outcomes included: in-hospital mortality; duration of postoperative ventilation; development of pulmonary infection; postoperative intensive care unit (ICU) length of stay; and hospital length of stay.
Statistical analysis
All analyses were conducted according to the presence or not of any transfusion during the perioperative period (RBC and/or FFP). In addition, patients were stratified according to the transfusion or not of RBC or FFP, to the transfusion of ≥3 units RBC or <3 units of RBC, and to transfusion both products (i.e., RBC and FFP), according to the tidal volume size used during intra-operative ventilation [≤7 mL/kg predicted body weight (PBW), 7–10 and >10 mL/kg PBW], and the resulting airway pressures (≤15, 15–20 and >20 cmH2O).
Time-to-event was defined as time from day of surgery to the event, reported in days. Cox proportional-hazards regression models were used to examine simultaneous effects of multiple covariates on outcomes. Patient’s data at the time of death, hospital discharge, or after 30 days were censored. Categorical outcome variables were tested for trend with the no-transfusion group as reference. The proportional-hazards assumption was assessed. Pairs of variables in the final model were tested for interaction. The effect of each variable in these models was assessed with the Wald test and reported using hazard ratio (HR) with 95% confidence interval (CI). Kaplan-Meier curves and log-rank tests were used to determine the univariate significance of the study variables.
A priori subgroup analyses were: (I) type of study (observational study vs. randomized controlled trial); (II) ASA score (<3 vs. ≥3 and for each level); (III) presence of risk factors for ARDS (yes or no and defined as pneumonia, shock, and sepsis); (IV) type of surgery (cardiac, abdominal, thoracic, and orthopedic); (V) age (<65 vs. ≥65 years); and (VI) sex (male vs. female).
Analyses were done with SPSS v.20 or R v.2.12.0. A two-sided P value <0.05 was considered significant.
Results
Search result and collection of individual patient data
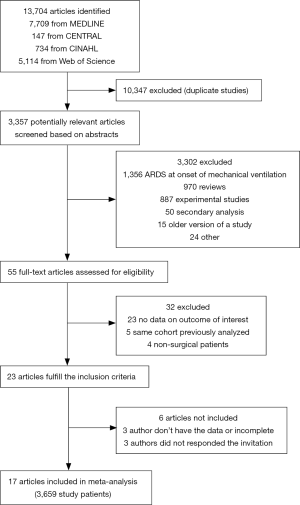
Three observational studies and 21 randomized controlled trials of ‘protective’ intra-operative ventilation were identified (12,16-38). We were not able to collect data from one observational study and six randomized controlled trials due to the following reasons: the corresponding author could not provide data (n=4) (32-35) or no contact could be made with the corresponding author (n=3) (36-38). The total enrollment based on the observational studies and randomized controlled trials for which individual patient data could be collected was 3,659 patients (Figure S1, Tables S1,S2) (9-25).
Full table
Full table
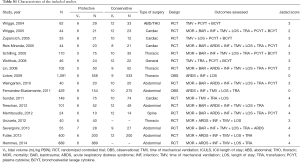
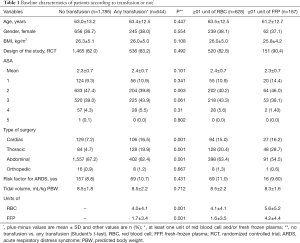
Table 1 details the distribution of demographic characteristics according to the presence or absence of transfusion and the number of units transfused. One thousand seventy-hundred eight-six patients did not receive any transfusion, and 644 patients received at least one transfusion. The distribution of the prevalence of risk factors for ARDS, the mean ASA, and the tidal volume was similar in the all groups.
Full table
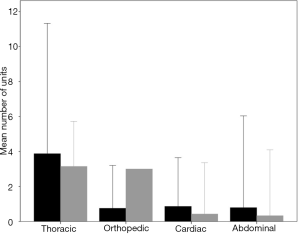
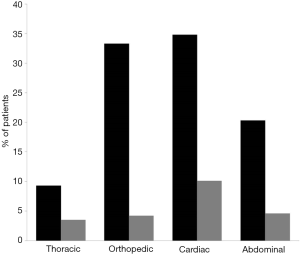
Six hundred twenty-eight patients received at least one unit of RBC and one hundred sixty-seven patients received at least one unit of FFP. The number of units of RBC and FFP according to type of surgery are shown in Figure S2 and the percentage of patients transfused with RBC or FFP according to the type of surgery are shown in Figure S3.
Predictors of transfusion
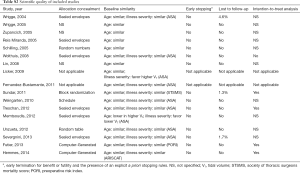
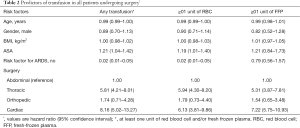
The probability of any transfusion was predicted by higher ASA, presence of shock, sepsis and/or pneumonia (risk factors for ARDS) and by the type of surgery (cardiac and thoracic). The probability of transfusion of at least one unit of RBC was also predicted by higher ASA, presence of risk factor for ARDS and by the type of surgery (cardiac and thoracic). The probability of transfusion of at least one unit of FFP was predicted solely by the type of surgery (cardiac and thoracic) (Table 2).
Full table
Primary outcome
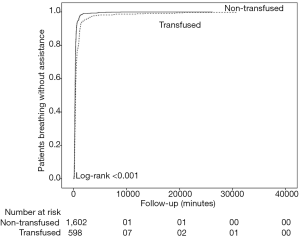
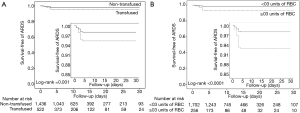
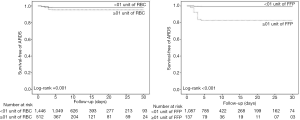
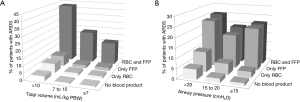
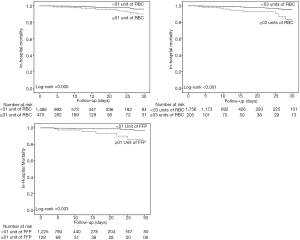
Postoperative ARDS occurred in 40 (7.2%) patients who received at least one blood product compared to 40 patients (2.5%) not transfused (adjusted HR, 2.32; 95% CI, 1.25–4.32; P=0.008) (Table 3, Figure 1A). Postoperative ARDS occurred in 29 (10.7%) patients who received more than 3 units RBC compared to 51 patients (2.7%) who received less than three units RBC (adjusted HR, 4.28; 95% CI, 2.23–8.21; P<0.0001) (Table 3, Figure 1B). The risk of ARDS was higher in patients who received at least one unit of FFP (adjusted HR, 3.52; 95% CI, 2.88–5.01; P=0.003), in patients who received at least one unit of RBC (adjusted HR, 2.36; 95% CI, 1.27–4.41; P=0.007) and in patients who received transfusion of both RBC and FFP (adjusted HR, 8.63; 95% CI, 6.09–14.65; P<0.001) (Table 3, Figure S4). Incidence of ARDS was higher in patients ventilated with tidal volumes >10 mL/kg PBW and receiving RBC and FFP compared to patients ventilated with tidal volume ≤7 mL/kg PBW receiving no transfusion (42.9% vs. 1.0%; P<0.001) (Figure 2A), and higher in patients with airway pressures >20 cmH2O and receiving RBC and FFP compared to patients with airway pressures ≤15 cmH2O receiving no transfusion (25% vs. 1.4%; P<0.001) (Figure 2B).

Full table
Secondary outcomes
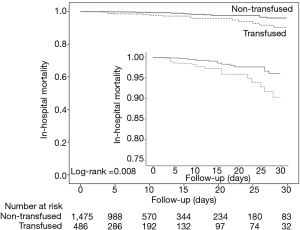
The crude mortality was lower in patients who were not transfused. However, after adjustment for baseline covariates, there was no difference in in-hospital mortality in patients who received or not any transfusion (Table 3, Figure 3), received any RBC, at least 3 units of RBC or any FFP (Table 3, Figure S5). There was no correlation between having received any transfusion and development of pulmonary infection (Table 3). Duration of postoperative mechanical ventilation and ICU length of stay was higher in patients who were transfused compared to those who were not transfused (828.6±2,094.3 vs. 382.9±809.3 minutes; P<0.001; and 2.5±5.5 vs. 1.1±3.9 days; P<0.001, respectively) (Figure S6). Hospital length of stay was similar in the two groups (14.6±15.8 vs. 15.5±17.5 days; P=0.265).
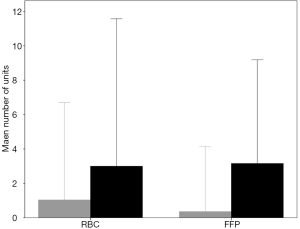
Patients who developed postoperative ARDS had received more units of RBC (3.0±4.3 vs. 1.0±2.8 units; P<0.001) and FFP (3.1±3.0 vs. 0.4±1.9 units; P<0.001) compared to those who did not developed postoperative ARDS (Figure S7). The percentage of patients who developed ARDS according to the number of units of RBC and FFP transfused are shown in Figure S8.

Pre-specified subgroup analyses
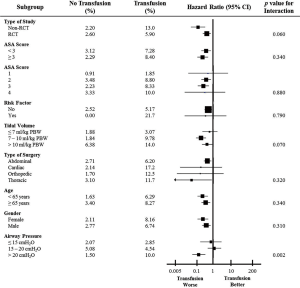
While there was trend for interaction on the effects of transfusion on primary outcome according to tidal volume size (P=0.070 for interaction), there was a significant interaction on primary outcome between transfusion and airway pressure used during surgery (P=0.002). There was no significant interaction according to the other prespecified subgroup analyses, like of ASA score (P=0.340), type of surgery (P=0.320), and sex (P=0.310) (Figure 4).
Discussion
This individual patient meta-analysis of 3,659 patients who received ventilation during general anesthesia for surgery from 17 clinical studies found evidence for a strong association between peri-operative transfusion of blood products and the incidence of postoperative ARDS independently from the severity of the disease. There was a trend for interaction between blood transfusion and the intra-operative tidal volume, and a significant interaction between blood transfusion and the airway pressure on the development of postoperative ARDS.
The results of this meta-analysis expand our knowledge on the association between transfusion and ARDS. A significantly higher odds of ARDS was found in critically ill medical patients who received blood transfusion (1). Higher risk of ARDS was also found in critically ill patients who received ventilation for more than 48 hours (5,39) and in trauma patients (4,40). Moreover, two randomized controlled trials, one in critically ill patients (41) and one in trauma patients (42) showed that a restrictive transfusion compared to liberal transfusion was associated with a lower incidence of ARDS. Thus, blood transfusion is associated with ARDS development in almost all subgroup of non-surgical patients.
Studies of transfusion and postoperative ARDS in surgical patients are scarce, and most studies were in cardiac surgery patients (9,43-45). In cardiac surgery, a restrictive transfusion strategy compared to a more liberal strategy resulted in similar rates of mortality and severe morbidity while use of blood products was less (43-45). Also, RBC transfusion is an independent risk factor for increased hospital length of stay in those patients undergoing cardiac surgery (46). The present meta-analysis of studies in general surgery patients, at least in part confirms the findings in cardiac surgery patients by showing a strong association between transfusion of blood products and postoperative ARDS, which holds for all types of surgery.
A two-hit hypothesis has been proposed for lung injury after transfusion (47). The first hit results in priming of granulocytes and adherence to the pulmonary endothelium. Then, the second hit is caused by mediators in the blood transfusion, which activate the endothelial cells and pulmonary granulocytes, resulting in pulmonary permeability and lung edema (3,47). Studies suggest that mechanical ventilation with higher tidal volume and higher airway pressures stretch the lung and results in priming of pulmonary granulocytes or endothelium making the lung susceptible to the second hit of blood transfusion (13,14,48).
Lung injury following transfusion can occur after any type of blood product (49). Plasma-rich products including platelets and FFP have been the most commonly implicated blood products (13,50,51) and FFP is usually associated with the most severe pulmonary reactions. We were not able to study effects of platelet transfusions due to problems in the report from corresponding authors. We did find in this study that the risk of lung injury was somewhat higher following FFP compared to RBC, but risk of RBC was also considerable. In line with this, several studies found a significant relationship between transfusion of RBC and pulmonary complications in general patients (41-46,52,53). Of note, we found that this relationship was dependent on the amount of transfusion, which has also been reported before (14,45,46). These data suggest that restrictive use of FFP and RBCs should be advocated whenever possible.
Since high tidal volume can be a first hit to the lung, it has been proposed that the use of ventilation with lower tidal volumes could be protective against lung injury induced by blood transfusion (3). Here we present for the first time the effect of blood transfusion on postoperative ARDS in patients ventilated with a variety of tidal volumes during general surgery. While there was only a trend for interaction between tidal volume and transfusion on ARDS development, a significant interaction between transfusion and airway pressure was found as a risk factor for ARDS. Thus, limiting tidal volume size, but especially airway pressure with intra-operative ventilation may mitigate the risk of lung injury induced by blood transfusion.
The stronger association between blood transfusion and airway pressure, as compared to transfusion and tidal volume, is remarkable. Recent studies suggest that increases in pulmonary vascular pressure as induced by mechanical ventilation are injurious. Cyclic changes in pressure in the tissue surrounding extra-alveolar vessels may be important in the pathogenesis of ventilator-induced lung injury (54). Also, increases in alveolar pressure are associated with increased damage in the vascular bed, promoting damage of endothelial cells and contributing to lung hemorrhage and permeability alterations induced by mechanical ventilation (55).
The present study has some limitations. First, from seven studies, not all original data could be retrieved (32-38). Second, we do not have information on some other factors that could have contributed to lung injury, including fluid balance and recruitment maneuvers. Third, we were not able to assess the reasons for blood transfusion. Fourth, different types of surgery were included. However, no interaction was found between type of surgery and primary outcome according to the prespecified subgroup analyses. Finally, blood transfusion may be collinear with other unmeasured aspects of severity of illness or treatment management.
Conclusions
In conclusion, this individual patient data meta-analysis suggests that transfusion of blood products (RBC and/or FFP) is strongly associated with postoperative ARDS in surgical patients. Non-protective ventilation settings, mainly high airway pressures, were found as a risk factor for postoperative ARDS together with blood transfusion.
Supplementary
Supplement I Online methods
Search strategy
Published studies were retrieved by directly contact with all corresponding authors from published studies on protective ventilation in surgery. This was achieved by the inclusion of the primary studies of a recent regular systematic review and meta-analysis by our group. In addition, the list of studies was updated to identify more recent studies. For this, two authors performed a computerized blinded search of MEDLINE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Web of Science, and Cochrane Central Register of Controlled Trials (CENTRAL) up to April 2013. The sensitive search strategy combined the following Medical Subject Headings and Keywords (protective ventilation OR lower tidal volume OR low tidal volume OR positive end-expiratory pressure OR positive end expiratory pressure OR PEEP). All reviewed articles and cross-referenced studies from retrieved articles were screened for pertinent information.
Selection of studies
All observational studies and randomized controlled trials (RCT) that reported outcomes in patients ventilated with lower VT in one arm and in those ventilated with higher VT in another were screened for inclusion. Key inclusion criteria are: (I) lower versus higher VT in each arm; (II) age >18 years; (III) patients undergoing any surgical procedures under general anesthesia and MV; and (IV) patients without ARDS at the onset of MV. Studies overlapping with other studies as indicated by the corresponding author were excluded. There was no restriction of study design or language.
Methodological quality assessment
Two investigators carried out the data extraction and quality assessment from all the retrieved published studies based on the full text articles. Discrepancies were resolved by consensus. In RCTs, we assessed allocation concealment, baseline similarity of groups (with regard to age, and severity of illness), early stopping of treatment, and lost to follow-up. Also, Jadad score was used to assess the quality of the RCTs.
Collection of individual patient data
Corresponding authors of the identified eligible published studies were contacted via email with a cover letter detailing the objectives of the collaborative meta-analysis, background information, and a datasheet for input of individual patient results for the project. The cover letter and the datasheet are shown below. The filled-out data templates was sent back to the principal investigator and further communication was mainly by email. Corresponding authors were also contacted about unpublished data to enlarge the clinical data pool.
Data management, security and validation
The same two investigators who performed the electronic database search also collected and assembled the individual patient data provided by the investigators. Data was accepted in any kind of electronic format (SPSS, STATA, Word document, Excel document, and Access document) and only the coordinators of the collaboration have direct access to the data. Both investigators performed data validation, checking the received data set for data entry mistakes and inconsistency. Differences was discussed and settled in consensus.
Mechanical ventilator and transfusion parameters
The corresponding author of each study was asked to fill the datasheet with MV parameters [plateau pressure (PPlateau), peak pressure (Ppeak), positive end-expiratory pressure (PEEP), respiratory rate (RR), inspired fraction of oxygen (FiO2), and minute-ventilation (MV)] and oxygenation parameters [partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2), pH, and PaO2/FiO2 ratio] obtained hourly during the procedure. Since some authors did not record the parameters hourly, we divided the measurements in three periods: (I) beginning of the surgery, defined as the parameters measured in the first hour of the procedure; (II) middle of the surgery, beginning of the surgery, defined as the parameters measured in the middle of the procedure (total time of procedure divided by two); and (III) end of the surgery, defined as the parameters measured in the last hour of the procedure. Since parameters in the end of the study can suffer influence of any lung injury developed during surgery and parameters the beginning of surgery doesn’t have sufficient time to induce changes in the lung, we chose to use the parameters in the middle of the surgery in the outcome analyses. A blood transfusion was defined as the infusion of red blood cells (RBCs) and/or fresh frozen plasma (FFP) in the perioperative period. The blood products were counted as units.
Primary and secondary outcomes
The primary outcome was ARDS development (according to the definition used by each author), where these data are available. The secondary clinical outcomes include: (I) in-hospital mortality, defined as any death during hospital stay; (II) barotrauma, according to the definition used by the author; (III) time of mechanical ventilation, defined as the time since initiation of mechanical ventilation and successful discontinuation; (IV) pulmonary infection, according to the definition used by the author; (V) ICU length of stay, defined as the time since admission in ICU and discharge of it; (VI) hospital length of stay, defined as the time since admission in hospital and discharge of it; (VII) RBC transfusion, defined as the amount of RBC in ml used during the follow-up; and (VIII) FFP transfusion, defined as the amount of FFP in ml used during the follow-up. Secondary laboratorial outcomes include: (I) levels of plasma interleukin (IL)-6, IL-8, IL-10, and TNF-α in pg/mL; and (II) levels of bronchoalveolar (BAL) IL-6, IL-8, and TNF-α in pg/mL.
Safety outcomes include: (I) PaO2 in mmHg; (II) PaCO2 in mmHg; (III) PaO2/FiO2 ratio; (IV) pH; and (V) incidence of acidosis, defined as pH <7.35.
Statistical analysis
Careful evaluation was performed to ensure completeness of data and to check consistency. Baseline characteristics of patients were presented separately for each trial and overall. Continuous variables were presented as mean and standard deviation (mean ± SD) or median and interquartile range [median[(IQR)] if not normally distributed. Binary and categorical variables were presented as frequencies and percentages [n (%)].
The formal statistical power depended on the number of patients ultimately included and was thus not fixed in advance. Post hoc calculations based strictly on the number of ARDS observed in each VT group indicated that the sample size we obtained provided 100% power to detect an interaction represented by a hazard ratio of 2.1 with a two-sided α of 0.05.
All analyses were conducted according to the presence or not of any transfusion during the perioperative period (RBC and/or FFP). In addition, patients were also stratified according to the transfusion or not of RBC or FFP, to the transfusion of more than 3 units RBC or less than three and to transfusion of RBC and FFP.
Time-to-event was defined as time from the day of surgery to the event. We used a Cox proportional-hazards regression model to examine simultaneously effects of multiple covariates on outcomes, censoring a patient’s data at the time of death, hospital discharge, or after 30 days (11). In all models, the categorical variables were tested for trend with the transfusion group as reference and the proportional-hazards assumption was assessed. A test for interaction between pairs of variables in the final model was performed. The effect of each variable in these models was assessed with the use of the Wald test and described by the HR with 95% CI.
The initial model included age, gender, type of study, BMI, type of surgery, ASA, tidal volume, highest PEEP, highest plateau pressure, highest compliance, and risk factors for ARDS. Variables with a P<0.2 in the univariate analysis are included in the multivariate regression. The final model was developed by dropping each variable in turn from the model and conducting a likelihood-ratio test to compare the full and the nested models. We used a significance level of 0.05 as the cutoff to exclude a variable from the model. Finally, the variable of transfusion was added to the model in order to test the resultant model against that without the variable. We constructed Kaplan-Meier curves and used log-rank test to determine the univariate significance of the study variables.
Time-course variables (e.g., repeated measures of ventilatory parameters, vital signs, oxygenation parameters and others) are also analyzed by a linear mixed model. Repeated-measures generalized linear model (GLM) was used to assess the time-interaction for ventilatory and oxygenation parameters during surgery. The model includes two factors: (I) study group (fixed factor), each level of the study group factor can have a different linear effect on the value of the dependent variable; (II) time as covariate, time is considered to be a random sample from a larger population of values, the effect is not limited to the chosen times.
Subgroup analyses were used to assess the effect of VT in the following pre-specified subgroups: (I) type of study (observational study vs. RCT); (II) ASA score (<3 vs. ≥3 and for each level); (III) presence of risk factors for ARDS (yes or no and defined as pneumonia, shock, and sepsis); (IV) tidal volume [≤7 mL/kg predicted body weight (PBW), 7–10 mL/kg PBW, and >10 mL/kg PBW]; (V) type of surgery (cardiac, abdominal, thoracic, and orthopedic); (VI) age (<65 vs. ≥65 years); (VII) gender (male vs. female); and plateau pressure (≤15 vs. 15–20 vs. >20 cmH2O). In data not normally distributed, analyses were performed after log10 transformation to permit the use of parametric statistics. If the data still differed significantly from normal even after log10 transformation, these data were analyzed by non-parametric tests.
All analyses were conducted with Review Manager v.5.1.1, SPSS v.20 and R v.2.12.0. For all analyses two-sided P values <0.05 were considered significant.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Khan H, Belsher J, Yilmaz M, et al. Fresh-Frozen Plasma and Platelet Transfusions Are Associated With Development of Acute Lung Injury in Critically Ill Medical Patients. Chest 2007;131:1308-14. [Crossref] [PubMed]
- Yilmaz M, Keegan MT, Iscimen R, et al. Toward the prevention of acute lung injury: Protocol-guided limitation of large tidal volume ventilation and inappropriate transfusion. Crit Care Med 2007;35:1660-6. [Crossref] [PubMed]
- Vlaar AP, Juffermans NP. Transfusion-related acute lung injury: a clinical review. Lancet 2013;382:984-94. [Crossref] [PubMed]
- Silverboard H, Aisiku I, Martin GS, et al. The role of acute blood transfusion in the development of acute respiratory distress syndrome in patients with severe trauma. J Trauma 2005;59:717-23. [PubMed]
- Gong MN, Thompson BT, Williams P, et al. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med 2005;33:1191-8. [Crossref] [PubMed]
- Baron DM, Hochrieser H, Posch M, et al. Preoperative anaemia is associated with poor clinical outcome in non-cardiac surgery patients. Br J Anaesth 2014;113:416-23. [Crossref] [PubMed]
- Shaw RE, Johnson CK, Ferrari G, et al. Blood transfusion in cardiac surgery does increase the risk of 5-year mortality: results from a contemporary series of 1714 propensity-matched patients. Transfusion 2014;54:1106-13. [Crossref] [PubMed]
- Wright SE, Snowden CP, Athey SC, et al. Acute lung injury after ruptured abdominal aortic aneurysm repair: the effect of excluding donations from females from the production of fresh frozen plasma. Crit Care Med 2008;36:1796-802. [Crossref] [PubMed]
- Vlaar AP, Hofstra JJ, Determann RM, et al. The incidence, risk factors, and outcome of transfusion-related acute lung injury in a cohort of cardiac surgery patients: a prospective nested case-control study. Blood 2011;117:4218-25. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Serpa Neto A, Cardoso SO, Manetta JA, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA 2012;308:1651-9. [Crossref] [PubMed]
- Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013;369:428-37. [Crossref] [PubMed]
- Vlaar AP, Wolthuis EK, Hofstra JJ, et al. Mechanical ventilation aggravates transfusion-related acute lung injury induced by MHC-I class antibodies. Intensive Care Med 2010;36:879-87. [Crossref] [PubMed]
- Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood 2012;119:1757-67. [Crossref] [PubMed]
- Serpa Neto A, Hemmes SN, de Abreu MG, et al. Protocol for a systematic review and individual patient data meta-analysis of benefit of so-called lung-protective ventilation settings in patients under general anesthesia for surgery. Syst Rev 2014;3:2. [Crossref] [PubMed]
- Wrigge H, Uhlig U, Zinserling J, et al. The effects of different ventilatory settings on pulmonary and systemic inflammatory responses during major surgery. Anesth Analg 2004;98:775-81. [Crossref] [PubMed]
- Wrigge H, Uhlig U, Baumgarten G, et al. Mechanical ventilation strategies and inflammatory responses to cardiac surgery: a prospective randomized clinical trial. Intensive Care Med 2005;31:1379-87. [Crossref] [PubMed]
- Zupancich E, Paparella D, Turani F, et al. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: A randomized clinical trial. J Thorac Cardiovasc Surg 2005;130:378-83. [Crossref] [PubMed]
- Reis Miranda D, Gommers D, Struijs A, et al. Ventilation according to the open lung concept attenuates pulmonary inflammatory response in cardiac surgery. Eur J Cardiothorac Surg 2005;28:889-95. [Crossref] [PubMed]
- Schilling T, Kozian A, Huth C, et al. The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesth Analg 2005;101:957-65. [Crossref] [PubMed]
- Wolthuis EK, Choi G, Dessing MC, et al. Mechanical ventilation with lower tidal volumes and positive end-expiratory pressure prevents pulmonary inflammation in patients without preexisting lung injury. Anesthesiology 2008;108:46-54. [Crossref] [PubMed]
- Lin WQ, Lu XY, Cao LH, et al. Effects of the lung protective ventilatory strategy on proinflammatory cytokine release during one-lung ventilation. Ai Zheng 2008;27:870-3. [PubMed]
- Licker M, Diaper J, Villiger Y, et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care 2009;13:R41-50. [Crossref] [PubMed]
- Weingarten TN, Whalen FX, Warner DO, et al. Comparison of two ventilatory strategies in elderly patients undergoing major abdominal surgery. Br J Anaesth 2010;104:16-22. [Crossref] [PubMed]
- Fernandez-Bustamante A, Wood CL, Tran ZV, et al. Intraoperative ventilation: incidence and risk factors for receiving large tidal volumes during general anesthesia. BMC Anesthesiol 2011;11:22-9. [Crossref] [PubMed]
- Sundar S, Novack V, Jervis K, et al. Influence of low tidal volume ventilation on time to extubation in cardiac surgical patients. Anesthesiology 2011;114:1102-10. [Crossref] [PubMed]
- Treschan TA, Kaisers W, Schaefer MS, et al. Ventilation with low tidal volumes during upper abdominal surgery does not improve postoperative lung function. Br J Anaesth 2012;109:263-71. [Crossref] [PubMed]
- Memtsoudis SG, Bombardieri AM, Ma Y, et al. The effect of low versus high tidal volume ventilation on inflammatory markers in healthy individuals undergoing posterior spine fusion in the prone position: a randomized controlled trial. J Clin Anesth 2012;24:263-9. [Crossref] [PubMed]
- Unzueta C, Tusman G, Suarez-Sipmann F, et al. Alveolar recruitment improves ventilation during thoracic surgery: a randomized controlled trial. Br J Anaesth 2012;108:517-24. [Crossref] [PubMed]
- Severgnini P, Selmo G, Lanza C, et al. Protective mechanical ventilation during general anesthesia for open abdominal surgery improves postoperative pulmonary function. Anesthesiology 2013;118:1307-21. [Crossref] [PubMed]
- PROVE Network Investigators for the Clinical Trial Network of the European Society of Anaesthesiology, Hemmes SN, Gama de Abreu M, et al. High versus low positive end-expiratory pressure during general anaesthesia for open abdominal surgery (PROVHILO trial): a multicentre randomised controlled trial. Lancet 2014;384:495-503. [Crossref] [PubMed]
- Chaney MA, Nikolov MP, Blakeman BP, et al. Protective ventilation attenuates postoperative pulmonary dysfunction in patients undergoing cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2000;14:514-8. [Crossref] [PubMed]
- Koner O, Celebi S, Balci H, et al. Effects of protective and conventional mechanical ventilation on pulmonary function and systemic cytokine release after cardiopulmonary bypass. Intensive Care Med 2004;30:620-6. [Crossref] [PubMed]
- Michelet P, D'Journo XB, Roch A, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology 2006;105:911-9. [Crossref] [PubMed]
- Lellouche F, Dionne S, Simard S, et al. High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology 2012;116:1072-82. [Crossref] [PubMed]
- Cai H, Gong H, Zhang L, et al. Effect of low tidal volume ventilation on atelectasis in patients during general anesthesia: a computed tomographic scan. J Clin Anesth 2007;19:125-9. [Crossref] [PubMed]
- Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139:530-7. [Crossref] [PubMed]
- Ahn HJ, Kim JA, Yang M, et al. Comparison between conventional and protective one-lung ventilation for ventilator-assisted thoracic surgery. Anaesth Intensive Care 2012;40:780-8. [PubMed]
- Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med 2004;32:1817-24. [Crossref] [PubMed]
- Croce MA, Tolley EA, Claridge JA, et al. Transfusions result in pulmonary morbidity and death after a moderate degree of injury. J Trauma 2005;59:19-23. [Crossref] [PubMed]
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care: Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340:409-17. [Crossref] [PubMed]
- Boffard KD, Riou B, Warren B, et al. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo controlled, double-blind clinical trials. J Trauma 2005;59:8-15. [Crossref] [PubMed]
- Koch CG, Li L, Sessler DI, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med 2008;358:1229-39. [Crossref] [PubMed]
- van de Watering L, Lorinser J, Versteegh M, et al. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion 2006;46:1712-8. [Crossref] [PubMed]
- Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA 2010;304:1559-67. [Crossref] [PubMed]
- Galas FR, Almeida JP, Fukushima JT, et al. Blood transfusion in cardiac surgery is a risk factor for increased hospital length of stay in adult patients. J Cardiothorac Surg 2013;8:54-60. [Crossref] [PubMed]
- Marik PE, Corwin HL. Acute lung injury following blood transfusion: expanding the definition. Crit Care Med 2008;36:3080-4. [Crossref] [PubMed]
- Vlaar AP, Binnekade JM, Prins D, et al. Risk factors and outcome of transfusion-related acute lung injury in the critically ill: a nested case-control study. Crit Care Med 2010;38:771-8. [Crossref] [PubMed]
- Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion 1985;25:573-7. [Crossref] [PubMed]
- Gajic O, Rana R, Mendez JL, et al. Acute lung injury after blood transfusion in mechanically ventilated patients. Transfusion 2004;44:1468-74. [Crossref] [PubMed]
- Gajic O, Dzik WH, Toy P. Fresh frozen plasma and platelet transfusion for nonbleeding patients in the intensive care unit: benefit or harm? Crit Care Med 2006;34:S170-3. [Crossref] [PubMed]
- Taylor RW, O’Brien J, Trottier SJ, et al. Red blood cell transfusions and nosocomial infections in critically ill patients. Crit Care Med 2006;34:2302-8. [Crossref] [PubMed]
- Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med 2008;36:2667-74. [Crossref] [PubMed]
- Hotchkiss JR Jr, Blanch L, Naveira A, et al. Relative roles of vascular and airspace pressures in ventilator-induced lung injury. Crit Care Med 2001;29:1593-8. [Crossref] [PubMed]
- Broccard AF, Hotchkiss JR, Suzuki S, et al. Effects of mean airway pressure and tidal excursion on lung injury induced by mechanical ventilation in an isolated perfused rabbit lung model. Crit Care Med 1999;27:1533-41. [Crossref] [PubMed]