Complications on minimally invasive oblique lumbar interbody fusion at L2–L5 levels: a review of the literature and surgical strategies
Introduction
Fusion techniques for cases of lumbar spinal instability are diverse, among which are: posterior lumbar interbody fusion and transforaminal lumbar interbody fusion (PLIF/TLIF), anterior lumbar interbody fusion (ALIF), lateral transpsoas lumbar interbody fusion (LLIF), and oblique lumbar interbody fusion (OLIF). LLIF includes direct lateral interbody fusion (DLIF) or extreme lateral interbody fusion (XLIF), which are same regarding transpsoas approach but different based on instruments used. Each technique has its advantages and disadvantages (1,2). OLIF is an emerging procedure that has progressively been used by spine surgeons (3,4). The retroperitoneal space allows direct access to the intervertebral space, thus avoiding injury to the paraspinal muscles, psoas muscle, and lumbar plexus (5,6). Recently, the complications most frequently associated with this technique have been reported (1,4,7-12). How to avoid such complications can be a major factor in deciding to use this procedure.
Advantages and disadvantages of the lumbar fusion techniques
It is of great importance to understand the pros and cons provided by the current surgical options for lumbar interbody fusion. The PLIF requires an extensive dissection of the paraspinal tissue as well as prolonged soft tissue retraction (13). Other complications include significant perioperative bleeding, postoperative radiculopathy secondary to the prolonged retraction of the dural sac, dural tear, and postoperative muscular atrophy caused by denervation during the approach (4,14). Harms et al. (15), described the TLIF as an alternative to PLIF. Subsequently, Foley et al. (16), published results applying a tubular retractor to PLIF, TLIF, and posterolateral fusion (16,17). Minimally invasive TLIF is associated with less damage to the paravertebral muscles, especially to multifidus muscle, shorter length of hospital-stay, lesser perioperative bleeding, and a reduced rate of procedure-related infections. However, it is also associated with greater perioperative radiation exposure as well as difficulty for lordosis restoration and coronal balance correction (2,16,18). ALIF was described by Capener (19) in 1932 and modified by Mayer (5) in 1997. This procedure offers an anterior access at L4–L5 and L5–S1 levels, allowing for a complete discectomy, better end-plate preparation, direct insertion of the cage, and adequate distraction for the development of lordosis. Moreover, this approach does not damage the posterior vertebral elements so that it can result in less postoperative axial back pain and a reduced possibility of adjacent segmental disease (ASD) (20). The complications described in the ALIF include damage to the abdominal viscera and anterior lumbar vessels, retrograde ejaculation, intestinal adhesions, and abdominal hernia (21-23). Ozgur et al. (24), described the XLIF in 2006. The intervertebral space is reached laterally using an expandable tubular retractor to be located in the retroperitoneal space through the psoas muscle. This type of fusion allows for the placement of a large cage in the intervertebral space, specifically in the apophyseal ring where the bone is strongest, improving the intervertebral height and the correction of the deformity (25). However, various studies have reported the rates of complications lie between 6.2% to 52% (26-28). Another study published the neurological complications associated with the minimally invasive lateral lumbar interbody fusion (MIS-LLIF), which include: plexus injuries (13.28%), sensory deficits (0–75%: permanent in 62%), motor deficits (0.7–33.6%), and anterior thigh pain (12.5–25%) (29). LLIF should be performed under transoperative neurophysiological monitoring to prevent lesions to the lumbar plexus while the tubular retractor is being placed through the psoas muscle (30). Lastly, it is not feasible to approach L5–S1 level and L4–L5 level with high iliac crest (28). The anterior oblique retroperitoneal approach was described by Mayer in 1997 (5). The OLIF is being used more extensively (31). Similar to the XLIF, with the OLIF, can be placed a significant graft or cage in the intervertebral space, restoring disc height and achieving indirect decompression including in patients with severe lumbar spinal stenosis without injuring the psoas muscle nor the lumbar plexus (32). Its utility for correcting deformity has also been reported (7). Additionally, the use of transoperative neurophysiologic monitoring is not necessary (3).
Complications associated with OLIF as reported on the literature
Silvestre et al. (1), reported complications in 179 patients upon employing the technique described by Mayer (5) with minimal variations. The most common complications were: incisional pain in 2.2% of patients, lower extremity symptoms due to sympathetic chain injury and vascular injuries were reported in 1.7%. Although the mean operative blood loss was 99.5±254.0 mL for all patients, averaging 56.8±131.3 mL per level, was observed a notoriously high operative blood loss in two of the three patients with a perioperative vascular injury. The systematic review performed by Li et al. (8), reported 1.5% intraoperative and 9.9% postoperative complications in 1,453 patients who underwent OLIF. Vascular injury was the most common intraoperative complication. In another study, Jin et al. (6) compared the results between minimally invasive direct lateral interbody fusion (MIS-DLIF) and minimally invasive oblique lumbar interbody fusion (MIS-OLIF). Forty-three patients were studied, and the only L4–L5 level was analyzed, 22 and 21 patients with MIS-DLIF and MIS-OLIF interventions, respectively. Complications were classified as procedure and non-procedure related, transitory and persistent. Relief within the first 30 days after the surgery was considered transient whereas persistent described those with presenting symptoms for more than 30 days after the surgery. The complications on MIS-DLIF were found in 13 of 22 patients, and 13.6% were classified as persistent complications. The complications on MIS-OLIF were found on 3 of 21 patients, 2 of which presented leg paresthesia and 1 with a local hematoma. Not one patient within this group presented a persistent complication. In a study directed by Abe et al. (10), in 155 patients, a complication incidence of 48.3% was reported. Intraoperative complications were reported in 44.5% of the cases. Postoperative complications were only seen in 4.7%. The most common complication was the endplate fracture followed by the transitory weakness of the psoas muscle and transient neurological symptoms, lesion to the segmental artery, and superficial surgical site infection, as well as 1 case of each of the following: ureter, radicular, and cauda equina injury. The complications reported by Kaiser et al. (33), in 51 patients were: 3.9% perioperative and 17.6% immediately postoperative. The perioperative complications described in this study were: vascular laceration and dural tear. Whereas the immediate postoperative complications were: transitory ileus, retroperitoneal hematoma, urinary tract infection, wound infection, and worsening of radiculopathy. Kim et al. (34), retrospectively evaluated the results of 29 patients operated with the OLIF technique. The reported complications were the subsidence of the cage on 8 of the 37 levels and four patients with alleviated lumbar plexopathy within four weeks after surgery. Injury to the sympathetic chain was documented using digital infrared thermal imaging, which was reported in four patients. There also exist reports on isolated intraoperative complications on OLIF. Chang et al. (35), described a case of a ventral dural tear during end-plate preparation and Lee et al. (36), reported an intraoperative ureter injury. With this information, interest should be taken in vascular, peritoneal, and urinary tract injury during OLIF (37). Table 1 summarized the complications reported in the literature.
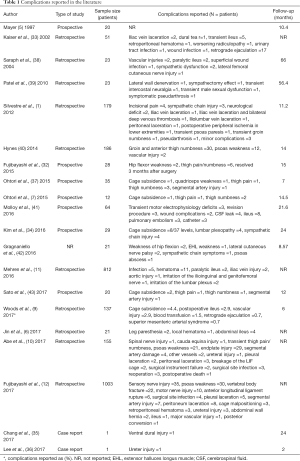
Full table
How to avoid complications in OLIF at L2–L5 levels
Preoperative planning
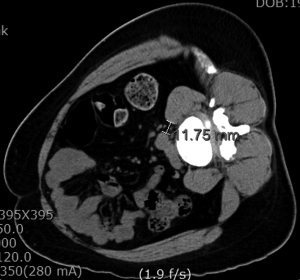
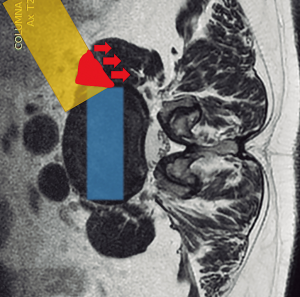
Magnetic resonance imaging (MRI) and computed tomography (CT) should be carefully reviewed before the procedure (3,6,9,44). The surgical planning guided by images is essential to appreciate the lumbar arterial and venous vessels, as well as their posterior and lateral migration on the contralateral side of the approach (28). MRI and CT scan in a right lateral decubitus position can provide accuracy towards the planning (44). The surgical corridor should be measured between the anterior border of the psoas muscle and the left lateral border of the anterior lumbar artery (aorta or left iliac artery) (Figure 1). Liu et al. (3), recommended opting for a different lumbar fusion technique when this distance is less than 1 cm, due to the risk of vascular damage and a greater retraction of the psoas muscle and lumbar plexus. Also, it is important to take into account the area of which can be obtained using a gentle retraction of the anterior belly of the psoas muscle (45). Therefore, we could get not only a fixed OLIF corridor from the CT/MRI images but also flexible OLIF corridor by retraction of the psoas muscle.
Incision and dissection of the abdominal wall
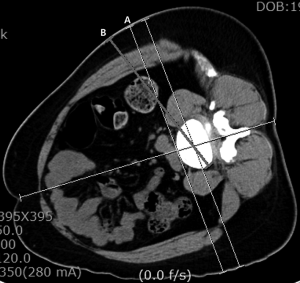
The patient should be placed in a right lateral position, to expose the left side, and fastened with adhesive drapes to avoid manipulation of the surgical field during the procedure. The mild flexion of the left hip will help relax the left psoas muscle (46). Molinares et al. (47), published that the patient in lateral decubitus over the flexed table and the time lapsed on this position are directly proportional to the postoperative neuropraxia. Therefore, is recommended to flex the surgical table during OLIF lightly. With the use of the fluoroscope and true lateral projections, it is advisable to project on the skin the anterior, posterior and, midpoint of the intervertebral space that will be fused (48). We consider that in this way guidance is given to the surgeon during the approach. The skin incision is localized anterior to the index level and can measure between 2.5 and 4 cm long per level, and also it can be performed in oblique or transversal fashion (1,9,11). The abdominal skin tends to be flexible and can be displaced with ease to reach two levels with an incision between 3 to 5 cm long (5,11,49). Incision have to be planned concerning preoperative images, taking advantage of the obliquity of the approach (33,43) (Figure 2). The patient´s wrong position, as well as a deficient fluoroscopic technique, can lead to a wrongly placed incision or the need to make a bigger approach. After correctly performing the incision, it is recommended to directly visualize the external and internal oblique muscles and the transverse muscle. The dissection should be blunt, with the same direction as the fibers in each muscular layer. Mirilas et al. (50), described four nerves that can be possibly found upon this dissection, these being the subcostal, iliohypogastric, ilioinguinal, and lateral femoral cutaneous nerves. With an adequate technique, these four nerves can be preserved. The iliohypogastric and ilioinguinal nerves tend to be seen underneath the internal oblique muscle. If they are found, they can be mobilized to avoid injury. It is also recommended to dissect the transversalis fascia as laterally as possible to evade the peritoneum. Extended muscular dissections should be prevented, and there should be precaution taken on the muscular closure of the abdominal wall as to avoid paresthesias, dysesthesias, and abdominal wall paresis after the procedure (3,51).
Dissection of the retroperitoneal space
The dissection of the retroperitoneal space should be done with the purpose of placing the tubular retractor over the disc. The anatomical limits of the retroperitoneal space are: the psoas muscle and the spine medially, peritoneum and abdominal viscera anteriorly, quadratus lumborum muscle and the iliac muscles posteriorly, diaphragm superiorly, and pelvis being the inferior limit, respectively (28). After identifying the peritoneum and exposing the retroperitoneal fatty tissue, a blunt dissection using an index finger is recommended; using back-and-forth and up-and-down movements until the anterior psoas border and intervertebral space is felt (Figure 3). The structures found during the trajectory towards this point should be retracted towards the midline. The adhesions that can occasionally be found between the peritoneum and the anterior border of the psoas muscle should be gently relieved (45). Some authors recommend palpating the lateral margin of the anterior longitudinal ligament as a reference of medial limit (11).
Psoas muscle mobilization
The anatomical corridor where the intervertebral disc is approached is limited by the anterior border of the psoas muscle and the left lateral border of the aorta or left iliac artery (44). The corridor area can be incremented with a minimal posterior retraction to the anterior belly of the psoas muscle (Figure 4). Davis et al. (45), reported an increment of the surgical corridor using this maneuver, obtaining a 59.60% at L2–L3, 43.96% at L3–L4, and 58.97% at L4–L5 percentages of increase, respectively. The L3–L4 level was reported as being the most extensive and the L4–L5 as being the narrowest. Thus, there should be precaution upon approaching this level. We suggest a meticulous dissection of the anterior belly of the psoas muscle that does not go beyond the median coronal plane as to avoid injury to the genitofemoral nerve (which runs on the anterolateral surface of the psoas muscle), lumbar plexus, and the muscle itself (3,11,42,52). Also, it should be taken into account that the prolonged retraction of the psoas muscle against the transverse process jeopardizes the lumbar plexus (42,52).
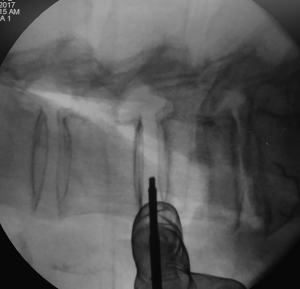
Tubular retractor placement
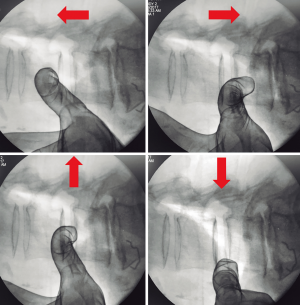
Tubular retractor should be placed on an oblique trajectory, and the vertex of the retractor should be centered to the intervertebral disc (44). To get this, the initial needle should be positioned correctly. We suggest that the initial needle should always be introduced protected underneath using the other hand’s index finger to avoid injuring structures during its trajectory (Figure 5). Recognizing the intervertebral space and discriminating between the disc and vertebral body structures should always be palpated using the index finger that protects the introduction of the initial needle, that way any potential laceration to the segmental lumbar artery can be avoided. The needle should be inserted into the junction of the anterior third with the middle third of the intervertebral space in the lateral fluoroscopic projection. This advice could facilitate the oblique placement of the tubular retractor and will also aid in the orthogonal maneuver for the cage placement. The lateral and anteroposterior projections guarantee security within the tubular retractor placement. Direct vision is necessary during this step; there should not be any structures left under the valves of the tubular retractor because of potential risk to the ureter, sympathetic chain, or vascular structures (Figure 6).
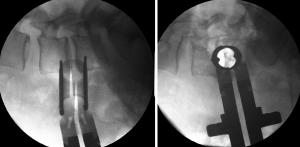
Vascular lesion
These can be presented due to lesions on the segmental lumbar vessels, main vessels such as renal vein or artery, or major vessels that rest on the anterior surface of the spine (53). The renal vessels are found anterior to the L1–L2 intervertebral space, and so the surgeon should be able to anticipate this situation when approaching with OLIF at higher levels such as L1–L2 and L2–L3 (3). The segmental lumbar arteries are direct branches of the aorta and thus supply blood to the caudal portion of the intervertebral foramen. These arteries run in a dorsolateral direction over the surface of the vertebral bodies. Orita et al. (53) described their location and angles based on magnetic resonance imaging. The angles on L1 and L3 are significantly acute (≤90 degrees) and substantially obtuse (>90 degrees) on L4 and L5. The lesion on the segmental vessels is associated to a laceration usually caused by the pin fixation of the tubular retractor. We recommend great caution upon the fixation of the tubular retractor at the L4–L5 space and if possible to avoid the fixation on L5 to prevent laceration to the iliolumbar vein (46). Moreover, the localization of these vessels should be prognosticated with the use of preoperative MRI. Palpation of the vertebral body with the initial needle while searching for the intervertebral body have to be avoided. Tubular retractor pin has to be inserted the most proximal to the endplates (53) (Figure 7). Damage to the great vessels that are found anteriorly to the lumbar spine is associated with its mobilization (3). This mobilization can be avoided through the use of a careful preoperative assessment through imaging and with the minimal medial exposure during the procedure (53).
Ureter lesion
Various authors coincide in noting that the level of greater risk for a ureter lesion is L2–L3 (45). The tubular retractor can overlap the ureter, specifically at superior lumbar levels. Therefore, ureter can be easily injured during any stage of the retroperitoneal corridor dissection and the placement of the tubular retractor. Fujibayashi et al. (54), proposed the use of dual-phase contrast-enhanced CT and reconstructed 3D images to know its preoperative anatomy. In this study, the ureter was localized anterior to the psoas muscle in 90.4% of the cases, and laterally to the vertebral body in 16% of the cases. Some surgical strategies to avoid a ureter injury could be the complete retraction of the retroperitoneal fatty tissue before starting the discectomy, the anterior mobilization of the ureter and, the inspection of the intervertebral space through the tubular retractor without seeing structures underneath the valves. Lastly, the possibility of a ureter lesion should be considered when faced with perioperative hematuria or non-specific signs and symptoms in the postoperative scenario, such as abdominal pain, fever, vomit, ileum, leukocytosis, or abdominal distention (36,54).
Sympathetic chain lesion
The sympathetic chain can be found in the anterior third of the vertebral body (11). However, in spite of it being a frequently described complication in the literature, there is no technical advice reported for the preservation of its integrity (1,6,8,34). Kim et al. (34), reported an incidence of sympathetic chain injury of 13.4% in 29 patients underwent OLIF L4–L5. We believe that sympathetic chain injury symptoms are underestimated because in some patients the thermal discrepancy in both legs is minimal and the symptoms are mild or reversible. Some authors suggest anterior mobilization after liberating the delicate communicating branches (11,42,52). The lesion to the sympathetic chain can be documented through physical exploration and the use of digital infrared thermal imaging (34). We recommend taking full advantage of the oblique vector that this approach offers so that the tubular retractor is placed posterior to the sympathetic chain and thus diminishing its manipulation.
Complications associated with the discectomy and the preparation of the endplates
A repeated fluoroscopic image-based control is recommended during the release of the contralateral annulus and should be done cautiously. Blunt surgical tools in this step are recommended to avoid a contralateral psoas muscle and lumbar plexus injury (10). Another technical advice is being able to understand the orthogonal maneuver that is executed in the discectomy, the endplates preparation, and the intervertebral cage placement. This maneuver refers to the 90-degree angle that is formed with the instruments when placed perpendicularly to the sagittal plane of the vertebral body. The maneuver initiates with the placement of the tubular retractor in an oblique fashion, and posteriorly during the endplates preparation, there is a 90-degree angle correction of the instruments (Figure 8). This confers to a proper control of the cage placement and the possibility of placing it more posteriorly than what the DLIF approach can offer (6) (Figure 9). Chang et al. (35), published a case where there was a ventral dural sac tear on an OLIF approach. This complication was due to disorientation in part of the surgeon upon fluoroscopic control during the endplates preparation as well as a failed orthogonal maneuver. Thus, the irruption to the spinal canal can be avoided by following the recommendations mentioned (10). In some cases, such as large disc herniation or disc herniation beyond the PLL indirect decompression might not be sufficient. There have been reports on endoscopic assistance for the OLIF to discectomy. The direct decompression through the conventional discectomy on OLIF is not recommended as it conveys a risk of spinal canal irruption (55,56).
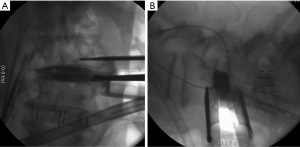
Subsidence
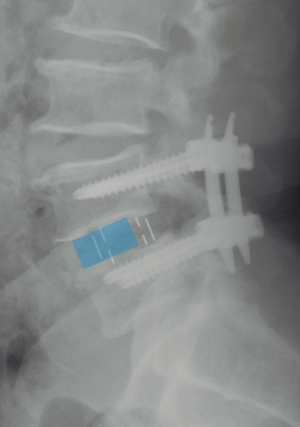
Currently, no literature specifically talks about how to prevent subsidence in OLIF. However, some reports describe that the incidence oscillates between 0.3% and 22% on XLIF. The subsidence can be solely radiographic, which is defined as a postoperative finding in images. When the subsidence is clinical, it is associated with axial pain and recurrent neurological symptoms related to the loss of indirect decompression. The iatrogenic subsidence occurs when the endplates are damaged during their preparation, placement of the cage or immediately after so, and it tends to be perioperative, associated with a deficient technique (57). Hence, subsidence depends on multiple factors related to the technique, implant material, and bone quality of the patient. Some specific situations reported in the literature are over-distraction, multilevel fusion, and small cages. Also, there have been reports citing that the superior lumbar vertebrae endplates are more susceptible to suffering subsidence in respect to the inferior vertebrae endplates and that the superior endplate is weaker than the inferior endplate in all lumbar vertebrae (25,57-60). When the cage is being placed, it is important to have in account that the endplate is most resistant peripherally and weaker centrally. In this way, large and wide cages that have bilateral contact with the periphery of the endplates have a diminished risk of subsidence (3,59). It is recommended to avoid an aggressive end-plate preparation (61). We suggest filling out the intervertebral space with contrast medium and taking fluoroscopic images on the anteroposterior and lateral projections with the objective of evidencing an adequate endplates preparation and also avoiding an aggressive preparation (Figure 10). At last, the cage placement on the middle third of the intervertebral space on the lateral projection of the fluoroscope, as well as the bilateral transpedicular fixation with screws can assist in avoiding the implant subsidence.
Conclusions
MIS-OLIF, for levels L2–L5, is a technique that has proven to have encouraging outcomes. However, there is still a need for high evidence-based, and larger sample sized studies to establish its feasibility entirely. Although its advantages concerning the lateral direct transpsoas (LLIF, DLIF o XLIF) technique are evident and the associated complications are few when they do present they can be catastrophic.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Silvestre C, Mac-Thiong JM, Hilmi R, et al. Complications and morbidities of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lumbar interbody fusion in 179 patients. Asian Spine J 2012;6:89-97. [Crossref] [PubMed]
- Mobbs RJ, Phan K, Malham G, et al. Lumbar interbody fusion: techniques, indications and comparison of interbody fusion options including PLIF, TLIF, MI-TLIF, OLIF/ATP, LLIF and ALIF. J Spine Surg 2015;1:2-18. [PubMed]
- Liu L, Liang Y, Zhang H, et al. Imaging anatomical research on the operative windows of oblique lumbar interbody fusion. PLoS One 2016;11:e0163452. [Crossref] [PubMed]
- Phan K, Maharaj M, Assem Y, et al. Review of early clinical results and complications associated with oblique lumbar interbody fusion (OLIF). J Clin Neurosci 2016;31:23-9. [Crossref] [PubMed]
- Mayer HM. A new microsurgical technique for minimally invasive anterior lumbar interbody fusion. Spine (Phila Pa 1976) 1997;22:691-9. [Crossref] [PubMed]
- Jin J, Ryu KS, Hur JW, et al. Comparative study of the difference of perioperative complication and radiologic results: MIS-DLIF (minimally invasive direct lateral lumbar interbody fusion) versus MIS-OLIF (minimally invasive oblique lateral lumbar interbody fusion). Clin Spine Surg 2018;31:31-6. [PubMed]
- Ohtori S, Mannoji C, Orita S, et al. Mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spinal kyphoscoliosis. Asian Spine J 2015;9:565-72. [Crossref] [PubMed]
- Li JX, Phan K, Mobbs R. Oblique lumbar interbody fusion: technical aspects, operative outcomes, and complications. World Neurosurg 2017;98:113-23. [Crossref] [PubMed]
- Woods KR, Billys JB, Hynes RA. Technical description of oblique lateral interbody fusion at L1-L5 (OLIF25) and at L5-S1 (OLIF51) and evaluation of complication and fusion rates. Spine J 2017;17:545-53. [Crossref] [PubMed]
- Abe K, Orita S, Mannoji C, et al. Perioperative complications in 155 patients who underwent oblique lateral interbody fusion surgery: perspectives and indications from a retrospective, multicenter survey. Spine (Phila Pa 1976) 2017;42:55-62. [Crossref] [PubMed]
- Mehren C, Mayer HM, Zandanell C, et al. The oblique anterolateral approach to the lumbar spine provides access to the lumbar spine with few early complications. Clin Orthop Relat Res 2016;474:2020-7. [Crossref] [PubMed]
- Fujibayashi S, Kawakami N, Asazuma T, et al. Complications associated with lateral interbody fusion: nationwide survey of 2998 cases during the first two years of its use in Japan. Spine (Phila Pa 1976) 2017;42:1478-84. [Crossref] [PubMed]
- Fan SW, Hu ZJ, Fang XQ, et al. Comparison of paraspinal muscle injury in one-level lumbar posterior inter-body fusion: modified minimally invasive and traditional open approaches. Orthop Surg 2010;2:194-200. [Crossref] [PubMed]
- Wang YP, An JL, Sun YP, et al. Comparison of outcomes between minimally invasive transforaminal lumbar interbody fusion and traditional posterior lumbar intervertebral fusion in obese patients with lumbar disk prolapse. Ther Clin Risk Manag 2017;13:87-94. [Crossref] [PubMed]
- Harms JG, Jeszenszky D. Die posteriore, lumbale, interkorporelle fusion in unilateraler transforaminaler technik. Oper Orthop Traumatol 1998;10:90-102. [Crossref] [PubMed]
- Foley KT, Holly LT, Schwender JD. Minimally invasive lumbar fusion. Spine (Phila Pa 1976) 2003;28:S26-35. [Crossref] [PubMed]
- Oppenheimer JH, DeCastro I, McDonnell DE. Minimally invasive spine technology and minimally invasive spine surgery: a historical review. Neurosurg Focus 2009;27:E9. [Crossref] [PubMed]
- Phan K, Rao PJ, Kam AC, et al. Minimally invasive versus open transforaminal lumbar interbody fusion for treatment of degenerative lumbar disease: systematic review and meta-analysis. Eur Spine J 2015;24:1017-30. [Crossref] [PubMed]
- Spondylolisthesis Capener N. Br J Surg 1932;19:374-86. [Crossref]
- Choi KC, Kim JS, Shim HK, et al. Changes in the adjacent segment 10 years after anterior lumbar interbody fusion for low-grade isthmic spondylolisthesis. Clin Orthop Relat Res 2014;472:1845-54. [Crossref] [PubMed]
- Phan K, Thayaparan GK, Mobbs RJ. Anterior lumbar interbody fusion versus transforaminal lumbar interbody fusion – systematic review and meta-analysis. Br J Neurosurg 2015;29:705-11. [Crossref] [PubMed]
- Kim JS, Choi WG, Lee SH. Minimally invasive anterior lumbar interbody fusion followed by percutaneous pedicle screw fixation for isthmic spondylolisthesis: minimum 5-year follow-up. Spine J 2010;10:404-9. [Crossref] [PubMed]
- Kim JS, Kang BU, Lee SH, et al. Mini-transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion augmented by percutaneous pedicle screw fixation: a comparison of surgical outcomes in adult low-grade isthmic spondylolisthesis. J Spinal Disord Tech 2009;22:114-21. [Crossref] [PubMed]
- Ozgur BM, Aryan HE, Pimenta L, et al. Extreme lateral interbody fusion (XLIF): a novel surgical technique for anterior lumbar interbody fusion. Spine J 2006;6:435-43. [Crossref] [PubMed]
- Lowe TG, Hashim S, Wilson LA, et al. A biomechanical study of regional endplate strength and cage morphology as it relates to structural interbody support. Spine (Phila Pa 1976) 2004;29:2389-94. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976) 2011;36:26-32. [Crossref] [PubMed]
- Tormenti MJ, Maserati MB, Bonfield CM, et al. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus 2010;28:E7. [Crossref] [PubMed]
- Regev GJ, Kim CW. Safety and the anatomy of the retroperitoneal lateral corridor with respect to the minimally invasive lateral lumbar intervertebral fusion approach. Neurosurg Clin N Am 2014;25:211-8. [Crossref] [PubMed]
- Epstein NE. Extreme lateral lumbar interbody fusion: Do the cons outweigh the pros? Surg Neurol Int 2016;7:S692-700. [Crossref] [PubMed]
- Cheng I, Acosta F, Chang K, et al. Point-counterpoint: the use of neuromonitoring in lateral transpsoas surgery. Spine (Phila Pa 1976) 2016;41:S145-51. [PubMed]
- Choma TJ, Mroz TE, Goldstein CL, et al. Emerging techniques in degenerative thoracolumbar surgery. Neurosurgery 2017;80:S55-S60. [Crossref] [PubMed]
- Fujibayashi S, Hynes RA, Otsuki B, et al. Effect of indirect neural decompression through oblique lateral interbody fusion for degenerative lumbar disease. Spine (Phila Pa 1976) 2015;40:E175-82. [Crossref] [PubMed]
- Kaiser MG, Haid RW Jr, Subach BR, et al. Comparison of the mini-open versus laparoscopic approach for anterior lumbar interbody fusion: a retrospective review. Neurosurgery 2002;51:97-103; discussion 103-5. [Crossref] [PubMed]
- Kim JS, Choi WS, Sung JH. 314 Minimally invasive oblique lateral interbody fusion for L4-L5: clinical outcomes and perioperative complications. Neurosurgery 2016;63:190-1. [Crossref] [PubMed]
- Chang J, Kim JS, Jo H. Ventral dural injury after oblique lumbar interbody fusion. World Neurosurg 2017;98:881.e1-881.e4. [Crossref] [PubMed]
- Lee HJ, Kim JS, Ryu KS, et al. Ureter injury as a complication of oblique lumbar interbody fusion. World Neurosurg 2017;102:693.e7-693.e14. [Crossref] [PubMed]
- Ohtori S, Orita S, Yamauchi K, et al. Mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for lumbar spinal degeneration disease. Yonsei Med J 2015;56:1051-9. [Crossref] [PubMed]
- Saraph V, Lerch C, Walochnik N, et al. Comparison of conventional versus minimally invasive extraperitoneal approach for anterior lumbar interbody fusion. Eur Spine J 2004;13:425-31. [Crossref] [PubMed]
- Patel NP, Birch BD, Dement SE, et al. The mini-open anterolateral approach for degenerative thoracolumbar disease. Clin Neurol Neurosurg 2010;112:853-7. [Crossref] [PubMed]
- Hynes RA. Oblique lateral interbody fusion (OLIF) technique and complications in 457 levels L1-S1. International Society for the Advancement of Spine Surgery 2014 Meeting. 2014. Available online: http://www.isass.org/abstracts/isass14_oral_posters/isass14-77-Oblique-Lateral-Interbody-Fusion-(OLIF)-Technique-and-Complications-in.html
- Molloy S, Butler JS, Benton A, et al. A new extensile anterolateral retroperitoneal approach for lumbar interbody fusion from L1 to S1: a prospective series with clinical outcomes. Spine J 2016;16:786-91. [Crossref] [PubMed]
- Gragnaniello C, Seex K. Anterior to psoas (ATP) fusion of the lumbar spine: evolution of a technique facilitated by changes in equipment. J Spine Surg 2016;2:256-65. [Crossref] [PubMed]
- Sato J, Ohtori S, Orita S, et al. Radiographic evaluation of indirect decompression of mini-open anterior retroperitoneal lumbar interbody fusion: oblique lateral interbody fusion for degenerated lumbar spondylolisthesis. Eur Spine J 2017;26:671-8. [Crossref] [PubMed]
- Molinares DM, Davis TT, Fung DA. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs: an MRI study. J Neurosurg Spine 2015. [Epub ahead of print]. [PubMed]
- Davis TT, Hynes RA, Fung DA, et al. Retroperitoneal oblique corridor to the L2-S1 intervertebral discs in the lateral position: an anatomic study. J Neurosurg Spine 2014;21:785-93. [Crossref] [PubMed]
- Hynes RA. Oblique lateral approach to the lumbar spine (L2-L5). In: Watkins III RG, Watkins IV RG, editors. Surgical approaches to the spine. New York: Springer; 2015;223-34.
- Molinares DM, Davis TT, Fung DA, et al. Is the lateral jack-knife position responsible for cases of transient neuropraxia? J Neurosurg Spine 2016;24:189-96. [Crossref] [PubMed]
- Mehren C, Korge A. Minimally invasive anterior oblique lumbar interbody fusion (OLIF). Eur Spine J 2016;25:471-2. [Crossref] [PubMed]
- Sofianos DA, Briseño MR, Abrams J, et al. Complications of the lateral transpsoas approach for lumbar interbody arthrodesis: a case series and literature review. Clin Orthop Relat Res 2012;470:1621-32. [Crossref] [PubMed]
- Mirilas P, Skandalakis JE. Surgical anatomy of the retroperitoneal spaces, part IV: retroperitoneal nerves. Am Surg 2010;76:253-62. [PubMed]
- Korenkov M, Rixen D, Paul A, et al. Combined abdominal wall paresis and incisional hernia after laparoscopic cholecystectomy. Surg Endosc 1999;13:268-9. [Crossref] [PubMed]
- Gragnaniello C, Seex KA. Anterior to psoas fusion of the lumbar spine. Neurosurg Focus 2013;35:Video 13.
- Orita S, Inage K, Sainoh T, et al. Lower lumbar segmental arteries can intersect over the intervertebral disc in the oblique lateral interbody fusion approach with a risk for arterial injury: radiological analysis of lumbar segmental arteries by using magnetic resonance imaging. Spine (Phila Pa 1976) 2017;42:135-42. [Crossref] [PubMed]
- Fujibayashi S, Otsuki B, Kimura H, et al. Preoperative assessment of the ureter with dual-phase contrast-enhanced computed tomography for lateral lumbar interbody fusion procedures. J Orthop Sci 2017;22:420-4. [Crossref] [PubMed]
- Heo DH, Choi WS, Park CK, et al. Minimally invasive oblique lumbar interbody fusion with spinal endoscope assistance: technical note. World Neurosurg 2016;96:530-6. [Crossref] [PubMed]
- Kim JS, Seong JH. Endoscope-assisted oblique lumbar interbody fusion for the treatment of cauda equina syndrome: a technical note. Eur Spine J 2017;26:397-403. [Crossref] [PubMed]
- Satake K, Kanemura T, Yamaguchi H, et al. Predisposing factors for intraoperative endplate injury of extreme lateral interbody fusion. Asian Spine J 2016;10:907-14. [Crossref] [PubMed]
- Hou Y, Luo Z. A study on the structural properties of the lumbar endplate: histological structure, the effect of bone density, and spinal level. Spine (Phila Pa 1976) 2009;34:E427-433. [Crossref] [PubMed]
- Le TV, Baaj AA, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976) 2012;37:1268-73. [Crossref] [PubMed]
- Grant JP, Oxland TR, Dvorak MF. Mapping the structural properties of the lumbosacral vertebral endplates. Spine (Phila Pa 1976) 2001;26:889-96. [Crossref] [PubMed]
- Uribe JS. Neural anatomy, neuromonitoring and related complications in extreme lateral interbody fusion: video lecture. Eur Spine J 2015;24 Suppl 3:445-6. [Crossref] [PubMed]


