Limitations and complications of minimally invasive spinal surgery in adult deformity
Introduction
Until recently, minimally invasive spine (MIS) techniques have been used mainly for the correction of coronal deformity due to an inability to impact the sagittal plane of patients with severe spinopelvic malalignment. A guideline for the role of MIS techniques in the treatment of adult spinal deformity has been previously published, reserving MIS for patients with either coronal Cobb angles less than 30 degrees or those with >30 degrees of deformity but little to no sagittal imbalance [sagittal vertical axis (SVA) <5 cm] (1). Yet the introduction of MIS anterior column realignment (ACR) with anterior longitudinal ligament release (ALLR) in 2013 has provided an improved ability to restore sagittal balance, and allows for similar radiographic outcomes to open surgery using MIS alone or a combination of hybrid techniques (2-5). While the indications for MIS have been greatly expanded, the limitations of these techniques need to be clearly understood. Certain forms of severe deformity are still best addressed with open techniques. New guidelines are currently being development to guide surgeons in the selection of appropriate surgical candidates.
Patient selection
Selecting the most appropriate approach for the patient can be challenging. Stand-alone lateral constructs should be reserved for patients with comorbidities preclusive of more complicated conventional or circumferential MIS approaches. Patients with any degree of spinal instability, or any degree of coronal or sagittal imbalance should not be treated with stand-alone lateral constructs. Patients with debilitating pain, progressive degenerative scoliosis with advanced age, and obvious medical co-morbidity that preclude prone positioning may be considered. These patients should be screened for osteopenia and osteoporosis however. The vertebral body end plate strength is greatly dependent on the bone density (6). Patient with osteoporosis or advanced osteopenia should not be considered for stand-alone lateral fusion, and may be best treated non-operatively or with limited decompressive surgeries. Perioperative treatment with recombinant human parathyroid hormone (teriparatide/Forteo) has been useful for preoperative augmentation of bone quality, with good outcomes in patients with poor bone mineral density scores. This drug not only has anti-resorptive but also osteoinductive properties that quickly re-establish good bone quality in patients requiring major spinal deformity surgery (7-12).
Indications for MIS lumbar interbody fusion (LIF) have been greatly expanded now to adult degenerative scoliosis, spondylosis and spondylolisthesis, trauma, degenerative disc disease, lumbar stenosis, and adjacent segment failure. MIS LIF has been associated with shorter odds ratio (OR) times, less blood loss, fewer complications, shorter hospital length of stay, and quicker recovery than open surgery (13,14). Long-term outcomes are generally favorable, with maintained improvements in patient-reported pain and function scores as well as radiographic parameters, including high rates of fusion.
Degenerative spine disease and deformity
Minimally invasive surgery was initially developed to address morbidity associated with traditional open spinal approaches. These techniques were first applied to the treatment of degenerative spinal disease. In particular MIS LIF has been effective for indirect foraminal and central decompression and fusion (15,16). More recently, these techniques have been applied to the treatment of degenerative deformity (i.e., adult spinal deformity). MIS LIF has been shown to effectively treat coronal deformity (13,17-19). However, the importance of sagittal imbalance on health related quality of life outcomes has recently been recognized (20). Positive sagittal balance can lead to higher energy requirements to stand and ambulate, leading to early fatigue, intolerance to standing, and walking with compensation through other joints. The impact of MIS LIF on sagittal plane correction has likewise been investigated, however the ability to correct lumbar lordosis and pelvic tilt via MIS LIF are modest at best if the ACR is also not included as part of the surgery (21).
While clinical outcomes data regarding MIS deformity correction is encouraging thus far (22), the main critique of MIS surgery in deformity correction has been its inability to improve sagittal balance to the same extent as traditional open surgery, leaving MIS options solely for mild sagittal or coronal deformity correction. Sagittal imbalance has been traditionally managed with Schwab’s posterior column shortening osteotomies, which have been reported to have at least 40% complication rate in adult spinal deformity (ASD) (23,24). ALLR with the use of hyperlordotic cages via the MIS lateral transpsoas approach has been shown to have similar radiographic and clinical outcomes as large posterior column osteotomies (PCOs), while at the same time minimizing complications of open surgery (25). Segmental lordosis after ALLR and ACR is increased by 14o when posterior elements are left intact. A facetectomy increases segmental lordosis restoration range to 21–27o. The spinous process can be resected along with bilateral facetectomy, achieving segmental lordosis restoration of up to 30o with a 30o hyperlordotic cage (26). These results are equivalent to open pedicle subtraction osteotomies (Figure 1).
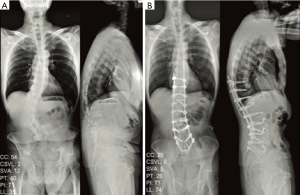
As increasing role of MIS LIF in moderate spinal deformity correction continues to be paved, it is important to keep in mind that the ultimate end goal should be to re-establish spinopelvic harmony, as it has been directly linked to a satisfactory postsurgical outcome as assessed by health related quality of life scores (20,27). Four basic radiographic targets to aim for in order to achieve spinopelvic harmony include: (I) sagittal vertical axis of <50 mm or T1–SI <0°; (II) pelvic tilt of <20°; (III) coronal Cobb angle <10; and (IV) lumbar lordosis pelvic incidence mismatch ±9° (20,28). These targets serve as the foundation for spinopelvic realignment in the sagittal and coronal plane, and even partial improvements of these parameters may translate to better clinical outcomes. Addition of ACR to the MIS armamentarium allows for greater MIS deformity correction previously not capable of with standard MIS techniques. And the severity of deformity dictates a particular MIS or hybrid technique approach (Table 1). It is important to note, however, that revision surgeries in patients with large dorsal fusion masses requiring significant pelvic incidence-lumbar lordosis (PI-LL) mismatch correction may not be amenable to MIS techniques alone, simply due to lack of available disc levels for ACR. In such cases, high grade open osteotomies may be necessary to restore spinopelvic harmony. Revision surgery with severe spinal deformity continues to be a limitation for MIS approaches alone.

Full table
Adjacent segment failure is a common complication encountered in patients with prior lumbar fusions. Correction of adjacent segment failure may involve additional posterior dissection, revision of existing instrumentation, and negotiation of scar tissue which may lead to increased risk of infection and spinal fluid leak. The MIS LIF is an option for treatment of adjacent segment failure. The lateral approach avoids traversing a scarred corridor, and allows placement of an intervertebral cage without the pitfalls of reoperation mentioned above. Additional fixation can be obtained using a lateral plate without the need to revise prior instrumentation. Literature regarding the use of the MIS LIF for adjacent segment failure is lacking, however there is literature regarding the use of this approach for revision lumbar surgery and total disc replacement (29-31).
Complications of MIS
Careful attention to detail throughout the perioperative period is crucial to reduce the risk of complications (32). Complications may arise from the result of inadequate preoperative planning. Meticulous review of preoperative imaging and assessment of neurovascular structures is necessary to avoid unintended injury. Transitional psoas may bring the femoral nerve closer to the posterior blade of the retractor, causing irreversible damage from nerve root ischemia or direct damage. Additionally, incorrect patient positioning may lead to difficulty accessing the L4–5 disk space, leading to increased risk of postoperative motor or sensory deficits.
Numbness, paresthesia, and weakness
Real-time directional electromyography (EMG) monitoring is crucial to minimize the chance of motor nerve injury (33). However, since sensory nerves cannot be monitored, understanding of and attention to regional anatomy is necessary to avoid sensory deficits. While injury can occur at any level of approach, the rate of femoral nerve injury is highest at the L4–5 segment. It is important to distinguish a true motor weakness along the femoral nerve distribution from pain limited weakness due to bruising of the psoas hip flexor from retraction. Reports of true motor weakness range from 3.4–23.7% (32,34,35). The rate of paresthesias following MIS LIF can range from 0.7–30% (32,34,36,37) and numbness has been reported in 8.3–42.4% (13,34,35). Commonly affected sensory nerves are the genitofemoral, lateral femoral cutaneous and anterior femoral cutaneous nerves. Most motor and sensory deficits are transient and recover, with 50% recovery at 90 days, and 90% recovery at 1 year (34).
Abdominal wall paresis and bowel perforation
Abdominal wall paresis, also referred to as a “pseudohernia”, may result from iatrogenic nerve injury during the initial dissection of the abdominal wall (38). This results in denervation, paresis, and bulging of the anterior abdominal wall. Associated signs and symptoms include swelling, pain, hyperesthesia, or other sensory abnormalities. It is imperative to rule out a true abdominal hernia in these instances, however in many cases spontaneous recovery can occur.
Lateral incisional hernia
This is a rare complication reported in approximately 1% of patients, and is mainly due to poor fascial closure technique (unpublished results). In our series of 303 patients, 3 were identified with incidental hernias during routine clinical follow-up. One of those patients, however, required hernia repair due to bowel incarceration.
Prevention of hernia formation is the key to complication avoidance. Full thickness closure of the transversalis fascia and muscle layers reduces the risk of incisional hernia, however the traversing nerves can be entrapped with blind sutures, leading to pseudohernia formation. Because of the small incisions used in lateral-MIS approaches, re-approximation of each individual layer may be challenging. In obese patients where visualization is limited, a layer by layer closure is not recommended. When operating near the iliac crest, leaving a small fascial cuff attached to the crest facilitates closure at the end of surgery. Fasciotomies in direct contact with bone are otherwise difficult to close. Lastly, un-breaking the table prior to closure allows a tension free closure and better approximation of tissue. Running or interrupted sutures may be used provided no residual fascial defects are left that would facilitate herniation of the peritoneal contents.
Colonic pseudo-obstruction and bowel perforation
Ogilvie’s syndrome (OS), or delayed ileus from colonic pseudo-obstruction can potentially lead to bowel perforation if not recognized in time, with associated mortality rate between 50% and 71% (39-41). It is not, however, a result of direct injury to bowel during surgery as it always presents itself in a delayed fashion. OS is clinically diagnosed as diminished gastric motility that does not resolve on its own in a matter of days. Radiographically it is characterized by dilatation of cecum greater than 9 cm and lack of mechanical obstruction on abdominal CT. Neostigmine, a acetylcholinesterase inhibitor, is rarely used in treating OS if more conservative measures fail (40,42).
Hardware-related complications and proximal junctional kyphosis (PJK)
There have been several reports of complications related to insertion of lateral interbody cages or lateral plates. Dua et al. reported a 15% rate of hardware-related complications in a series of 13 patients (43). These cases consisted of two atraumatic coronal plane fractures at L4/5 in the first 6 weeks of the postoperative period. Le et al. demonstrated hardware-related complication rate of 5.9% in a series of 101 patients (44,45). Included were three hardware failures and three vertebral body fractures. All cases presented with recurrent back pain except one, which was identified incidentally. The mechanism of hardware failure is unclear, but may involve cage subsidence with a fixed angle screw, resulting in increased force at an area of stress concentration, violation of the endplate during preparation or screw insertion, or incorrect placement of the hardware lock nuts (43-46).
PJK resulting in failure does not improve with the use of MIS techniques, and in fact, may increase when MIS is combined with hybrid PCOs. In a recent unpublished study by Uribe et al., PJK developed in 35.5% of patients, of which 16.1% progressed to proximal junctional failure (PJF). The incidence of PJK increased with addition of PCO (46.2% vs. 27.8%). While the mechanism for this is unknown, overcorrection in elderly patients may be a risk factor. Recent literature suggests that the goal SVA may be more liberal in the elderly than +/− 5 cm (47-49). Also, there was a higher PJK rate when the upper-instrumented vertebra was located at T10–L1 vs. L2–L4, suggesting location of instrumentation may also be contributory. Further research needs to be performed to pinpoint the exact cause of PJK and PJF in both MIS and open spinal surgery.
Subsidence
As with any technique used for lumbar fusion, subsidence of the cage can occur at either endplate. The subsequent progressive deformity and compression of neural elements can lead to a loss of indirect decompression and reduced chance of successful fusion (50,51).
In a study that included 140 patients and 238 levels fused in the lumbar spine, we recently found subsidence to be present in 14.3% of the cases, and in 8.8% of the total levels fused at a mean follow-up of 9.6 months (44). Only 2.1% of the patients had symptomatic subsidence, however, subsidence appears to correlate with construct length.
Subsidence appears largely correlated with cage size, as there was a 14.1% rate of subsidence with cages smaller than 18 mm, compared to a 1.9% rate of subsidence in cages larger than 22 mm. As such, the largest interbody cage should be used whenever feasible.
Rhabdomyolysis
Rhabdomyolysis is a rare, but known, complication of spinal surgery. Rhabdomyolysis leading to acute renal failure after MIS LIF has also been previously reported (52). Patients who are morbidly obese or procedures with a prolonged operative time are at an increased risk for this complication.
Contralateral psoas hematoma in LIF
Contralateral psoas hematoma is suspected to occur from segmental vessel injury during contralateral annulotomy (53). Preoperative axial and sagittal magnetic resonance imaging (MRI) series should be evaluated to determine whether there are traversing segmental vessels across the contralateral disc space. Contralateral leg weakness can occur as a result of this complication due to femoral nerve compression causing symptomatic neuropraxia. Prompt evacuation is recommended to prevent permanent injury.
Discussion
Due to recent rapid advancement in MIS techniques such as ACR, introduction of hyperlordotic lateral cages, and expandable transforaminal lumbar interbody fusion (TLIF) and LIF titanium cages, surgical indications have greatly expanded to include trauma, degenerative disc disease, spondylosis with instability, lumbar stenosis, spondylolisthesis, adjacent segment failure, and adult degenerative scoliosis with and without sagittal imbalance. MIS techniques have recently been applied to patients with sagittal imbalance or coronal deformity, and studies have confirmed its equivalence to open surgery, with the advantage of reduced blood loss and complications associated with open osteotomies. Yet limitations for the use of these techniques in complex spine surgery still exist, and their application must be evaluated on a case by case scenario. Patients with a prior large fusion mass, significantly increased PI-LL mismatch and large PT are difficult candidates for MIS correction. While MIS can be utilized at adjacent segment levels, such patients may still require a high-grade Schwab osteotomy to accomplish a good sagittal realignment. One of the major disappointments of MIS in spinal deformity surgery is its inability to reduce PJK and PJF rates. Further studies are needed to address this topic.
The benefits of MIS are becoming increasingly obvious in the literature; however, surgeons must remember to exercise judgment when electing to use these techniques over more traditional open procedures.
Conclusions
Recent advancements in spine surgery have reduced the limitations of MIS significantly. MIS does however have unique limitations, some of which are patient specific. Complications, while vastly different from open surgery, exist and must be understood to improve both radiographic and clinical patient outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: JS Uribe is a consultant for NuVasive, Inc. and receives consultation fees for his input on product design. The other authors have no conflicts of interest to declare.
References
- Mummaneni PV, Shaffrey CI, Lenke LG, et al. The minimally invasive spinal deformity surgery algorithm: a reproducible rational framework for decision making in minimally invasive spinal deformity surgery. Neurosurg Focus 2014;36:E6. [Crossref] [PubMed]
- Akbarnia BA, Mundis GM Jr, Moazzaz P, et al. Anterior column realignment (ACR) for focal kyphotic spinal deformity using a lateral transpsoas approach and ALL release. J Spinal Disord Tech 2014;27:29-39. [Crossref] [PubMed]
- Leveque JC, Yanamadala V, Buchlak QD, et al. Correction of severe spinopelvic mismatch: decreased blood loss with lateral hyperlordotic interbody grafts as compared with pedicle subtraction osteotomy. Neurosurg Focus 2017;43:E15. [Crossref] [PubMed]
- Manwaring JC, Bach K, Ahmadian AA, et al. Management of sagittal balance in adult spinal deformity with minimally invasive anterolateral lumbar interbody fusion: a preliminary radiographic study. J Neurosurg Spine 2014;20:515-22. [Crossref] [PubMed]
- Mundis GM Jr, Turner JD, Kabirian N, et al. Anterior Column Realignment has Similar Results to Pedicle Subtraction Osteotomy in Treating Adults with Sagittal Plane Deformity. World Neurosurg 2017;105:249-56. [Crossref] [PubMed]
- Oxland TR, Lund T. Biomechanics of stand-alone cages and cages in combination with posterior fixation: a literature review. Eur Spine J 2000;9 Suppl 1:S95-101. [Crossref] [PubMed]
- Chaudhary N, Lee JS, Wu JY, et al. Evidence for Use of Teriparatide in Spinal Fusion Surgery in Osteoporotic Patients. World Neurosurg 2017;100:551-6. [Crossref] [PubMed]
- Cho PG, Ji GY, Shin DA, et al. An effect comparison of teriparatide and bisphosphonate on posterior lumbar interbody fusion in patients with osteoporosis: a prospective cohort study and preliminary data. Eur Spine J 2017;26:691-7. [Crossref] [PubMed]
- Ebata S, Takahashi J, Hasegawa T, et al. Role of Weekly Teriparatide Administration in Osseous Union Enhancement within Six Months After Posterior or Transforaminal Lumbar Interbody Fusion for Osteoporosis-Associated Lumbar Degenerative Disorders: A Multicenter, Prospective Randomized Study. J Bone Joint Surg Am 2017;99:365-72. [Crossref] [PubMed]
- Fischer CR, Hanson G, Eller M, et al. A Systematic Review of Treatment Strategies for Degenerative Lumbar Spine Fusion Surgery in Patients With Osteoporosis. Geriatr Orthop Surg Rehabil 2016;7:188-96. [Crossref] [PubMed]
- Kaliya-Perumal AK, Lu ML, Luo CA, et al. Retrospective radiological outcome analysis following teriparatide use in elderly patients undergoing multilevel instrumented lumbar fusion surgery. Medicine (Baltimore) 2017;96:e5996. [Crossref] [PubMed]
- Seki S, Hirano N, Kawaguchi Y, et al. Teriparatide versus low-dose bisphosphonates before and after surgery for adult spinal deformity in female Japanese patients with osteoporosis. Eur Spine J 2017;26:2121-7. [Crossref] [PubMed]
- Dakwar E, Cardona RF, Smith DA, et al. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus 2010;28:E8. [Crossref] [PubMed]
- Youssef JA, McAfee PC, Patty CA, et al. Minimally invasive surgery: lateral approach interbody fusion: results and review. Spine (Phila Pa 1976) 2010;35:S302-11. [Crossref] [PubMed]
- Carreon LY, Puno RM, Dimar JR 2nd, et al. Perioperative complications of posterior lumbar decompression and arthrodesis in older adults. J Bone Joint Surg Am 2003;85-A:2089-92. [Crossref] [PubMed]
- Okuda S, Miyauchi A, Oda T, et al. Surgical complications of posterior lumbar interbody fusion with total facetectomy in 251 patients. J Neurosurg Spine 2006;4:304-9. [Crossref] [PubMed]
- Anand N, Rosemann R, Khalsa B, et al. Mid-term to long-term clinical and functional outcomes of minimally invasive correction and fusion for adults with scoliosis. Neurosurg Focus 2010;28:E6. [Crossref] [PubMed]
- Tormenti MJ, Maserati MB, Bonfield CM, et al. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus 2010;28:E7. [Crossref] [PubMed]
- Wang MY, Mummaneni PV. Minimally invasive surgery for thoracolumbar spinal deformity: initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus 2010;28:E9. [Crossref] [PubMed]
- Schwab F, Patel A, Ungar B, et al. Adult spinal deformity-postoperative standing imbalance: how much can you tolerate? An overview of key parameters in assessing alignment and planning corrective surgery. Spine (Phila Pa 1976) 2010;35:2224-31. [Crossref] [PubMed]
- Le TV, Vivas AC, Dakwar E, et al. The effect of the retroperitoneal transpsoas minimally invasive lateral interbody fusion on segmental and regional lumbar lordosis. ScientificWorldJournal 2012;2012:516706. [PubMed]
- Mundis GM, Akbarnia BA, Phillips FM. Adult deformity correction through minimally invasive lateral approach techniques. Spine (Phila Pa 1976) 2010;35:S312-21. [Crossref] [PubMed]
- Glassman SD, Hamill CL, Bridwell KH, et al. The impact of perioperative complications on clinical outcome in adult deformity surgery. Spine (Phila Pa 1976) 2007;32:2764-70. [Crossref] [PubMed]
- Yadla S, Maltenfort MG, Ratliff JK, et al. Adult scoliosis surgery outcomes: a systematic review. Neurosurg Focus 2010;28:E3. [Crossref] [PubMed]
- Deukmedjian AR, Dakwar E, Ahmadian A, et al. Early outcomes of minimally invasive anterior longitudinal ligament release for correction of sagittal imbalance in patients with adult spinal deformity. ScientificWorldJournal 2012;2012:789698. [PubMed]
- Uribe JS, Harris JE, Beckman JM, et al. Finite element analysis of lordosis restoration with anterior longitudinal ligament release and lateral hyperlordotic cage placement. Eur Spine J 2015;24 Suppl 3:420-6. [Crossref] [PubMed]
- Lafage V, Schwab F, Vira S, et al. Spino-pelvic parameters after surgery can be predicted: a preliminary formula and validation of standing alignment. Spine (Phila Pa 1976) 2011;36:1037-45. [Crossref] [PubMed]
- Deukmedjian AR, Ahmadian A, Bach K, et al. Minimally invasive lateral approach for adult degenerative scoliosis: lessons learned. Neurosurg Focus 2013;35:E4. [Crossref] [PubMed]
- Leary SP, Regan JJ, Lanman TH, et al. Revision and explantation strategies involving the CHARITE lumbar artificial disc replacement. Spine (Phila Pa 1976) 2007;32:1001-11. [Crossref] [PubMed]
- Patel AA, Brodke DS, Pimenta L, et al. Revision strategies in lumbar total disc arthroplasty. Spine (Phila Pa 1976) 2008;33:1276-83. [Crossref] [PubMed]
- Wagner WH, Regan JJ, Leary SP, et al. Access strategies for revision or explantation of the Charite lumbar artificial disc replacement. J Vasc Surg 2006;44:1266-72. [Crossref] [PubMed]
- Knight RQ, Schwaegler P, Hanscom D, et al. Direct lateral lumbar interbody fusion for degenerative conditions: early complication profile. J Spinal Disord Tech 2009;22:34-7. [Crossref] [PubMed]
- Uribe JS, Vale FL, Dakwar E. Electromyographic monitoring and its anatomical implications in minimally invasive spine surgery. Spine (Phila Pa 1976) 2010;35:S368-74. [Crossref] [PubMed]
- Cummock MD, Vanni S, Levi AD, et al. An analysis of postoperative thigh symptoms after minimally invasive transpsoas lumbar interbody fusion. J Neurosurg Spine 2011;15:11-8. [Crossref] [PubMed]
- Pimenta L, Oliveira L, Schaffa T, et al. Lumbar total disc replacement from an extreme lateral approach: clinical experience with a minimum of 2 years' follow-up. J Neurosurg Spine 2011;14:38-45. [Crossref] [PubMed]
- Bergey DL, Villavicencio AT, Goldstein T, et al. Endoscopic lateral transpsoas approach to the lumbar spine. Spine (Phila Pa 1976) 2004;29:1681-8. [Crossref] [PubMed]
- Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion: an analysis of 600 cases. Spine (Phila Pa 1976) 2011;36:26-32. [Crossref] [PubMed]
- Dakwar E, Le TV, Baaj AA, et al. Abdominal wall paresis as a complication of minimally invasive lateral transpsoas interbody fusion. Neurosurg Focus 2011;31:E18. [Crossref] [PubMed]
- Maloney N, Vargas HD. Acute intestinal pseudo-obstruction (Ogilvie's syndrome). Clin Colon Rectal Surg 2005;18:96-101. [Crossref] [PubMed]
- Ponec RJ, Saunders MD, Kimmey MB. Neostigmine for the treatment of acute colonic pseudo-obstruction. N Engl J Med 1999;341:137-41. [Crossref] [PubMed]
- Vanek VW, Al-Salti M. Acute pseudo-obstruction of the colon (Ogilvie's syndrome). An analysis of 400 cases. Dis Colon Rectum 1986;29:203-10. [Crossref] [PubMed]
- Hooten KG, Oliveria SF, Larson SD, et al. Ogilvie's syndrome after pediatric spinal deformity surgery: successful treatment with neostigmine. J Neurosurg Pediatr 2014;14:255-8. [Crossref] [PubMed]
- Dua K, Kepler CK, Huang RC, et al. Vertebral body fracture after anterolateral instrumentation and interbody fusion in two osteoporotic patients. Spine J 2010;10:e11-5. [Crossref] [PubMed]
- Le TV, Baaj AA, Dakwar E, et al. Subsidence of polyetheretherketone intervertebral cages in minimally invasive lateral retroperitoneal transpsoas lumbar interbody fusion. Spine (Phila Pa 1976) 2012;37:1268-73. [Crossref] [PubMed]
- Le TV, Smith DA, Greenberg MS, et al. Complications of lateral plating in the minimally invasive lateral transpsoas approach. J Neurosurg Spine 2012;16:302-7. [Crossref] [PubMed]
- Disch AC, Knop C, Schaser KD, et al. Angular stable anterior plating following thoracolumbar corpectomy reveals superior segmental stability compared to conventional polyaxial plate fixation. Spine (Phila Pa 1976) 2008;33:1429-37. [Crossref] [PubMed]
- Banno T, Togawa D, Arima H, et al. The cohort study for the determination of reference values for spinopelvic parameters (T1 pelvic angle and global tilt) in elderly volunteers. Eur Spine J 2016;25:3687-93. [Crossref] [PubMed]
- Hasegawa K, Okamoto M, Hatsushikano S, et al. Normative values of spino-pelvic sagittal alignment, balance, age, and health-related quality of life in a cohort of healthy adult subjects. Eur Spine J 2016;25:3675-86. [Crossref] [PubMed]
- Yukawa Y, Kato F, Suda K, et al. Normative data for parameters of sagittal spinal alignment in healthy subjects: an analysis of gender specific differences and changes with aging in 626 asymptomatic individuals. Eur Spine J 2018;27:426-32. [Crossref] [PubMed]
- Closkey RF, Parsons JR, Lee CK, et al. Mechanics of interbody spinal fusion. Analysis of critical bone graft area. Spine (Phila Pa 1976) 1993;18:1011-5. [Crossref] [PubMed]
- Kozak JA, Heilman AE, O'Brien JP. Anterior lumbar fusion options. Technique and graft materials. Clin Orthop Relat Res 1994.45-51. [PubMed]
- Dakwar E, Rifkin SI, Volcan IJ, et al. Rhabdomyolysis and acute renal failure following minimally invasive spine surgery: report of 5 cases. J Neurosurg Spine 2011;14:785-8. [Crossref] [PubMed]
- Beckman JM, Vincent B, Park MS, et al. Contralateral psoas hematoma after minimally invasive, lateral retroperitoneal transpsoas lumbar interbody fusion: a multicenter review of 3950 lumbar levels. J Neurosurg Spine 2017;26:50-4. [Crossref] [PubMed]


