Heart-lung interactions during mechanical ventilation: the basics
Introduction
The first demonstration of heart lung interactions was published by Hales et al. in 1733 demonstrating circulatory variations during respiratory cycles in mares using glass tube manometers (1). Over the years our understanding of heart and lung interactions have improved and helped inform our clinical decision making particularly in the arena of critical care. Thus, the purpose of this review should help critical care physicians understand the basics of heart and lung interaction under negative pressure ventilation and the effects of positive pressure ventilation on this complex system.
Before discussing mechanical effects of heart lung interactions, it is important to understand that spontaneous breathing is exercise (2). It consumes oxygen, requires increased blood flow and produces carbon dioxide. Although beyond the scope of this discussion on the basics of heart-lung interactions, if mechanical ventilation relieves the respiratory muscles, then both whole body oxygen consumption will decrease, allowing a limited and cardiac output to sub serve the other metabolic demands it faces. Whereas weaning from mechanical ventilation induced cardiovascular stress and can induce both heart failure and pulmonary edema.
The heart and lung interplay occurs due to their anatomic location: they both occupy the same thoracic cavity, connected via blood vessels. Because of this housing of the heart within the thorax, the heart can be described as a “pressure chamber within a pressure chamber”. Pressure changes within the thoracic cavity during the respiratory cycle affect the pressure systems to the heart and from the heart to the extra-thoracic spaces but do not alter the intrathoracic vascular relationships. The reasons for these differential effects is because flow through the circuit is determined by driving pressures (pressure gradients) within that circuit. The pressure gradient for blood flow is different for the arterial and venous sides of the circulation. For venous return the pressure gradient is from the mean systemic reservoirs, referred to as mean circulatory filling pressure (Pmcf) to the right atrium and for the arterial circuit from the left ventricle into the arterial tree. Since intrathoracic pressure (ITP) is the surrounding pressure for the heart, right atrial pressure (Pra) relative to right ventricular (RV) filling is best quantified as Pra minus ITP, referred to as transmural (across the wall) pressure. Similarly, left ventricular (LV) ejection pressure is estimates as arterial pressure minus ITP. Clearly, both transmural Pra and transmural LV pressure vary with changes in ITP while neither the upstream venous driving pressure, referred to as mean systemic filling pressure (Pmsf), nor arterial pressure are affected by isolated changes in ITP. Therefore, these changes in ITP can markedly alter these pressure gradients by altering transmural right atrial and transmural LV pressures both cyclically during breathing and during the steady state if ITP is kept increased or decreased relative to atmosphere. However, the pulmonary arterial to left atrial pressure gradient is not altered by changes in ITP because that entire circuit is within the thorax. Thus, the systemic circulation can be profoundly altered by ITP changes whereas the pulmonary circulation is immune unless lung volumes also change.
Right heart and determinants of preload
Preload is RV end-diastolic wall stress. Whether changes in RV end-diastolic volume actually change wall stress is a matter of speculation. Clearly, under resting condition in a normal heart, RV end-diastolic volume varies over a wide range with minimal changes in transmural RV pressure. Thus, under resting conditions the force of RV ejection is remarkably constant, explaining why fluid resuscitation invariably increases RV end-systolic volume as well and end-diastolic volume. However, as described above, the pressure gradient for venous return to the RV from the circulation is Pmsf relative to Pra. The venous system carries about 70% of the blood volume in the body. Most of this venous volume is housed in vessels that sense as their outside surrounding pressure atmospheric pressure. If one could withdraw blood from an adynamic circulation, one would see that Pmsf would decrease to zero despite having more than half the blood still remaining in the circulation. That is because that amount of blood in the circulation below the volume that causes increases in vascular pressure, fill the vascular space not by stretch the vessels but by causing them to have a conformational expansion from a collapsed state. Once distend any further increase in intravascular volume causes Pmcf to increase. The amount of blood in the systemic circulation below this pressure inflection point is called the unstressed volume of the circulation, and the amount above it is called the stressed volume. Any further intravascular volume increase above this stressed volume point will result in an increase in Pmsf along the venous compliance relationship. Thus, Pmsf is determined by the stressed volume causing positive transmural pressure against the vessel wall. Whereas total circulating blood volume define both stressed and unstressed volume. Elastic recoil against this venous vessel pressure provides the driving force for blood to flow towards the heart (3-5). Since different vascular beds have different amounts of unstressed volume, altering blood flow distribution toward low unstressed volume circuits (e.g., muscle) or increasing venomotor tone (e.g., increased sympathetic tone, vasopressors) will increase Pmsf increasing the upstream pressure for venous return (4,6). Finally, respiratory cycles by selectively altering Pra can directly influence this pressure gradient as well (3,7,8) (Figure 1).
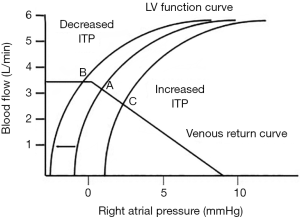
Pra is the back pressure to venous return and opposes Pmsf. Dynamic change in Pra during the ventilatory cycle causes reciprocal changes in venous flow rates (9). Pra was thought to be influenced by compliance of the right atrium as well as changes in transmural pressure during respiratory cycles, such that with RV failure and right atrial dilation, small changes in intravascular volume will induce proportionally greater increases in Pra than otherwise seen. Transmural pressure for the right atrium is considered to be the pressure gradient between the Pra and the pressure surrounding the outside of the myocardium. In the absence of pericardial pathology such as tamponade, this would be effectively pleural pressure (Ppl) (10). During inspiration Ppl decreases, and for a constant transmural Pra causing Pra to decrease (11). This spontaneous inspiration-induced decrease in Pra causes an immediate increase in venous return increasing RV end-diastolic volume and then RV stroke volume on the next beat. While during expiration, Ppl becomes less negative causing Pra to increase to its end-expiratory value and venous return decreases slightly.
While the effect ITP swings on Pra is well documented (12,13) the role of transmural Pra pressure on RV performance has been called into question. For example, Tyberg et al. (10) measured pericardial pressure and Pra in patients during fluid loading prior to cardiac surgery demonstrated and found that although Pra increased, pericardial pressure increased equally, such that transmural Pra remained constant. Similarly, Lansdorp et al. (14) measured juxtacardiac pleural and pericardial pressures using air-filled balloons in 20 post cardiopulmonary bypass patients at different tidal volumes during mechanical ventilation. They found the transmural pressure of the right atrium (derived by Pra—pericardial pressure) did not change with increases in the tidal volume (from 4, 6, 8 and 10 mL/kg). Both these clinical studies are consistent with the statement that over the normal physiologic range, RV filling occurs below its own unstressed volume.
Positive-pressure ventilation reverses the effect on Pra during the respiratory cycle, increasing Pra during inspiration and decreasing it during expiration. Airway, Pra, pericardial and pleural pressures all increase with increasing tidal volumes and ITP in a linear fashion (14). With mechanical inflation of the lungs during inspiration, ITP and Pra increases. This in turn decreases driving pressure for venous return and RV end-diastolic volume (8,15).
The understanding of this concept of how changing ITP alters the pressure gradient for venous return and thus cardiac output is vital for clinicians taking care of ventilated patients, particularly in the setting of hypovolemia. The pressure gradient driving blood from venous reservoirs to the heart is normally only 4–8 mmHg (16). Since the resistance of venous return (RVR) is very low, such a small pressure gradient is adequate to drive 100% of the cardiac output back to the heart each minute. Thus, small increases in positive end-expiratory pressure (PEEP) can cause relatively large decreases in preload and overall cardiac output. This effect must be mitigated by increasing Pmsf by increasing stressed volume or increasing vessel tone (17). Furthermore, in an animal model Katira et al. (18) demonstrated the effect of large tidal volumes and zero PEEP on sharp decreases in Pra, RV end-diastolic volume, and since it also created an increased RV ejection pressure (increased pulmonary vascular resistance by large tidal inspirations) developed progressive cor pulmonale. By simply adding 10 cmH2O PEEP and lowering tidal volumes these detrimental effects were minimized.
Other than its direct effect on the heart, both positive and negative pressure inspiration can influence preload by increasing venous return from abdominal vasculature due to increasing abdominal pressure owing to diaphragmatic excursion (19,20). In the case of negative pressure ventilation, the amount of blood returning to the heart may be limited by ITP itself as Pra becomes sub-atmospheric, causing the great vessels to collapse due to as they enter the thoracic inlet creating a flow-limited segment (21).
During mechanical ventilation this effect is important in mitigating the decrease in RV preload caused by an increasing ITP (22-24) by increasing Pmsf and thus minimizing the detrimental effects of increased Pra on the pressure gradient for venous return. This was demonstrated in fluid resuscitated post-cardiac surgery patients using a 25-second inspiratory hold and 20 mmHg of PEEP (25). In these 42 patients, cardiac outputs remained unchanged during inspiratory holds and progressively increasing levels of PEEP despite rising Pra because intraabdominal pressure increased to a similar amount allowing intra-abdominal venous compartments to proportionally increase their upstream venous pressures.
Left heart and determinants of afterload
Afterload is the force resisting ventricular ejection (26). In the absence of aortic valve pathology this resisting force is determined by aortic pressure, arterial elastance and overall arterial resistance (27). The higher the aortic elastance, or stiffness of the arterial tree, the less it can accommodate pulsatile blood flow from the left ventricle without increasing arterial pressure. In cases of chronic hypertension and aortic calcification, a resultant increased elastance markedly increases LV afterload and impairs LV stroke volume in response to exercise while increasing long term mortality.
During negative pressure respiration, inspiration leads to decreasing Ppl and increasing transmural pressure LV ejection pressure. This hinders LV contraction by the increased LV afterload causing LV end-systolic volume to increase on the very first beat (17). The opposite is true for expiration and forced expiration, where increasing ITP and Ppl and decreasing transmural pressure decrease afterload decreasing LV end-systolic volume for the same arterial pressure. In healthy adults during spontaneous breathing, these negative swings in ITP have little effect on LV systolic performance because the normal LV can easily sustain ejection against small increases in afterload (28). However, if the decreases in ITP are marked (e.g., upper airway obstruction, laryngeal edema, obstructive sleep apnea or head and neck tumors), inspiration occurs against a closed airway and ITP markedly decreases. This causes large immediate increases in LV afterload and venous return, increasing intrathoracic fluid content, and if severe and/or prolonged promoting pulmonary edema (28,29).
During mechanical ventilation particularly when high PEEP or large tidal volumes are employed, inspiration increases Ppl, decrease LV transmural pressure and decreases LV afterload aiding in LV ejection even if arterial pressure also increases (30). This is especially notable in patients with congestive heart failure. However, these increased LV stroke volume effects are limited by the associated decrease in venous return, as described above. Plus, if lung volume increases, then pulmonary vascular resistance also increases impeding RV ejection. Thus, the combination of increase ITP increase decrease pressure gradient for venous return plus increased lung volume-induced increase in pulmonary vascular resistance may create a critically low output state. Still, this effect of positive pressure ventilation is helpful in certain disease states such as left-sided systolic heart failure (31) especially if lung volume increases are minimized. A probable cause of LV failure during ventilator weaning must be the associated increased LV afterload induced by the phasic decreases in ITP with each spontaneous breath with its obligatory increase in myocardial O2 consumption. This weaning-associated LV failure may be a primary cause of failure to wean in critically ill ventilator-dependent patients (32,33).
Right heart, lung volumes and determinants of RV afterload
The right heart has been described more as a flow generator than a pressures generator owing to its ejection at lower pressure into a more compliant pulmonary vasculature (11,34). During mechanical ventilation, changes in ITP are the main determinants of changes in LV afterload. However, for the right ventricle, these changes have minimal effects on the right ventricle because the entire pulmonary vasculature is within the intrathoracic compartment and affected equally by changing ITP. However, change in lung volume associated with ventilation can markedly alter pulmonary vascular resistance and elastance as well as pulmonary arterial pressures due to changing zonal conditions, all of which are the primary determinants of RV afterload (30,35).
During inspiration, the increasing lung volume causes the pulmonary vasculature to distend, increasing its compliance and minimizing increased RV stroke volume-induced increases in RV afterload. Because the RV has less contractile reserve than the LV, ITP swings and afterload during the respiratory cycle have a greater effect on the RV than the LV (36). This concept becomes especially important in disease states like acute respiratory distress syndrome (ARDS) where hypoxic vasoconstriction can increase afterload and potentially cause the RV to fail (30). Recruitment maneuvers that open collapsed alveolar units will reduce overall pulmonary vascular impedance and resistance, promoting effective RV ejection.
In mechanical ventilation, inspiration-associated over-inflation of lung volume will increase pulmonary vascular resistance increasing RV afterload. Thus, RV ejection can be impeded during inspiration if large tidal volumes are used (37). This effect appears to be mitigated in smaller lung-protective tidal volume routinely used in ventilated patients and the use of lower levels of PEEP. Changes in Ppl affect lung West zones 1, where alveolar pressure (Palv) exceeds pulmonary artery pressure (Ppa) and zone 2 where Pa exceed Palv but Palv exceeds pulmonary venous pressure (Ppv) (38). During mechanical inspiration delivery of positive Ppl can create more zone 1 and 2 areas, altering pulmonary blood flow to zone 3 areas, increasing resistance and RV afterload and causing both an increased dead space ventilation and potential increased shunt blood flow (11). This effect is due primarily to the increases in lung volume caused by positive pressure breathing, not by the increases in Palv themselves. For example, if tidal volume is kept constant but chest wall compliance markedly reduced, no change in pulmonary blood flow occurs. Similarly, in subjects with decreased lung compliance (e.g., ARDS) the effects of increased Palv are often blunted (39). However, ARDS is usually associated with increased pulmonary arterial pressure independent of mechanical ventilatory strategies, thus RV afterload may still be increased due to hypoxic vasoconstriction from pulmonary edema and lung injury rather than a high Palv exceeding Ppa.
RV and LV linked dynamics: interdependence
The LV and RV pump blood in parallel but are also connected in series. Hence, LV end diastolic volume correlates with RV preload (40). Ventricular interdependence occurs by virtue of the ventricles sharing a septum, their location within a fixed volume pericardial space and their anatomical orientation of free wall myofibril inter-associations. The left ventricle has a thick spherical shape with a helical orientation while the right ventricle is wrapped around the left with a thin free wall. The myocardial fibers of the LV mostly contribute to the septum. RV systolic function is dependent on this septum and the RV free wall to LV free wall fiber connections (41). This is likely the case in cardiovascular dysfunction associated with ventricular dyssynchrony (e.g., single ventricular pacing, bundle branch blocks or post myocardial infarction). LV dyssynchrony affects the systolic and diastolic performance of both ventricles (42). Yamaguchi et al. determined that LV contraction contributes 20–40% of RV systolic pressure and RV contraction contributes 4–10% of LV systolic pressure (43).
Occupying a pericardial space and sharing a septum also affects biventricular lusitropy (i.e., active relaxation and diastolic filing). With limited space to expand, increased filling of one ventricle decreases the diastolic compliance of the other (44). This is apparent in cases of pulmonary embolism with RV failure wherein massive RV dilation causes LV end-diastolic volume to collapse. Spontaneous inspiration, by increasing venous return and RV end-diastolic volume also cause similar though markedly less impressive changes in LV end-diastolic volume over the ventilatory cycle independent of LV filling pressure. Although over a sum of heart beats, mean RV stroke volume should equal mean LV stroke volume, there are impressive beat-to-beat variations caused by the effect of ITP on both ventricles. Under normal conditions, pulmonary vasculature low elastance and high capacitance allows for the pulmonary vasculature to accommodate RV stroke volume variations without much change in pulmonary artery pressure (45). So spontaneous breathing increases RV stroke volume and decreases LV stroke volume, which reverse on exhalation but steady state cardiac output is relatively constant
Comparing ventilation modes
The effect of mechanical ventilation on the heart and hemodynamics essentially related to how each mode of ventilation alters mean and changing ITP and lung volume (46). Different ventilator modes can affect patients in similar ways if their impact on ITP and lung volume is similar. This holds true despite marked differences in waveforms or differences in complete or partial respiratory support as long as tidal volumes and PEEP remain similar (47-49). Pressure control ventilation has been compared to volume-control ventilation demonstrating unchanged cardiac outputs if tidal volumes are matched and higher cardiac outputs if tidal volumes are lower (50,51). In 25 acute lung injury patients, the hemodynamic effects of pressure-controlled and volume-controlled ventilation modes were similar provided mean Paw was similar across modes (52). Singer et al. demonstrated in ventilated patients that it was lung hyperinflation and not Paw that decreased cardiac output (53).
Conclusions
Ventilation is a ubiquitous phenomenon and its effects on cardiovascular function a mandatory result. By understand the simple individualized determinants of their interactions, one can deconvolute the more complex presentations of advance ventilatory modes, levels of cardiovascular and pulmonary insufficiency and how to interpret their findings and treat those patients in the most efficient manner as to minimize detrimental heart-lung interactions, while preserving the beneficial ones.
Acknowledgements
Funding: This work was supported in part by NIH grants HL07820, GM126811 and NR013912.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Sette P, Dorizzi RM, Azzini AM. Vascular access: an historical perspective from Sir William Harvey to the 1956 Nobel prize to Andre F. Cournand, Werner Forssmann, and Dickinson W. Richards. J Vasc Access 2012;13:137-44.
- Pinsky MR. Breathing as exercise: the cardiovascular response to weaning from mechanical ventilation. Intensive Care Med 2000;26:1164-6. [Crossref] [PubMed]
- Magder S. Volume and its relationship to cardiac output and venous return. Crit Care 2016;20:271. [Crossref] [PubMed]
- Berger D, Moller PW, Weber A, et al. Effect of PEEP, blood volume, and inspiratory hold maneuvers on venous return. Am J Physiol Heart Circ Physiol 2016;311:H794-806. [Crossref] [PubMed]
- Magder S, De Varennes B. Clinical death and the measurement of stressed vascular volume. Crit Care Med 1998;26:1061-4. [Crossref] [PubMed]
- Bloch A, Berger D, Takala J. Understanding circulatory failure in sepsis. Intensive Care Med 2016;42:2077-9. [Crossref] [PubMed]
- Magder S. The classical Guyton view that mean systemic pressure, right atrial pressure, and venous resistance govern venous return is/is not correct. J Appl Physiol (1985) 2006;101:1533. [Crossref] [PubMed]
- Pinsky MR. Instantaneous venous return curves in an intact canine preparation. J Appl Physiol Respir Environ Exerc Physiol 1984;56:765-71. [PubMed]
- Moller PW, Winkler B, Hurni S, et al. Right atrial pressure and venous return during cardiopulmonary bypass. Am J Physiol Heart Circ Physiol 2017;313:H408-20. [Crossref] [PubMed]
- Tyberg JV, Taichman GC, Smith ER, et al. The relationship between pericardial pressure and right atrial pressure: an intraoperative study. Circulation 1986;73:428-32. [Crossref] [PubMed]
- Grubler MR, Wigger O, Berger D, et al. Basic concepts of heart-lung interactions during mechanical ventilation. Swiss Med Wkly 2017;147. [PubMed]
- Wise RA, Robotham JL, Summer WR. Effects of spontaneous ventilation on the circulation. Lung 1981;159:175-86. [Crossref] [PubMed]
- Morgan BC, Abel FL, Mullins GL, et al. Flow patterns in cavae, pulmonary artery, pulmonary vein, and aorta in intact dogs. Am J Physiol 1966;210:903-9. [Crossref] [PubMed]
- Lansdorp B, Hofhuizen C, van Lavieren M, et al. Mechanical ventilation-induced intrathoracic pressure distribution and heart-lung interactions*. Crit Care Med 2014;42:1983-90. [Crossref] [PubMed]
- Kilburn KH. Cardiorespiratory effects of large pneumothorax in conscious and anesthetized dogs. J J Appl Physiol 1963;18:279-83. [Crossref] [PubMed]
- Nanas S, Magder S. Adaptations of the peripheral circulation to PEEP. Am Rev Respir Dis 1992;146:688-93. [Crossref] [PubMed]
- Magder SA, Lichtenstein S, Adelman AG. Effect of negative pleural pressure on left ventricular hemodynamics. Am J Cardiol 1983;52:588-93. [Crossref] [PubMed]
- Katira BH, Giesinger RE, Engelberts D, et al. Adverse Heart-Lung Interactions in Ventilator-induced Lung Injury. Am J Respir Crit Care Med 2017;196:1411-21. [Crossref] [PubMed]
- Fessler HE, Brower RG, Wise RA, et al. Effects of positive end-expiratory pressure on the canine venous return curve. Am Rev Respir Dis 1992;146:4-10. [Crossref] [PubMed]
- Takata M, Robotham JL. Effects of inspiratory diaphragmatic descent on inferior vena caval venous return. J Appl Physiol (1985) 1992;72:597-607. [Crossref] [PubMed]
- Guyton AC, Lindsey AW, Abernathy B, et al. Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol 1957;189:609-15. [Crossref] [PubMed]
- Matuschak GM, Pinsky MR, Rogers RM. Effects of positive end-expiratory pressure on hepatic blood flow and performance. J Appl Physiol (1985) 1987;62:1377-83. [Crossref] [PubMed]
- Chihara E, Hashimoto S, Kinoshita T, et al. Elevated mean systemic filling pressure due to intermittent positive-pressure ventilation. Am J Physiol 1992;262:H1116-21. [PubMed]
- Takata M, Wise RA, Robotham JL. Effects of abdominal pressure on venous return: abdominal vascular zone conditions. J Appl Physiol (1985) 1990;69:1961-72. [Crossref] [PubMed]
- van den Berg PC, Jansen JR, Pinsky MR. Effect of positive pressure on venous return in volume-loaded cardiac surgical patients. J Appl Physiol (1985) 2002;92:1223-31. [Crossref] [PubMed]
- Reichek N, Wilson J, St John Sutton M, et al. Noninvasive determination of left ventricular end-systolic stress: validation of the method and initial application. Circulation 1982;65:99-108. [Crossref] [PubMed]
- Walley KR. Left ventricular function: time-varying elastance and left ventricular aortic coupling. Crit Care 2016;20:270. [Crossref] [PubMed]
- Buda AJ, Pinsky MR, Ingels NB Jr, et al. Effect of intrathoracic pressure on left ventricular performance. N Engl J Med 1979;301:453-9. [Crossref] [PubMed]
- Cozanitis DA, Leijala M, Pesonen E, et al. Acute pulmonary oedema due to laryngeal spasm. Anaesthesia 1982;37:1198-9. [Crossref] [PubMed]
- Vieillard-Baron A, Matthay M, Teboul JL, et al. Experts' opinion on management of hemodynamics in ARDS patients: focus on the effects of mechanical ventilation. Intensive Care Med 2016;42:739-49. [Crossref] [PubMed]
- Pinsky MR. Cardiovascular issues in respiratory care. Chest 2005;128:592S-7S. [Crossref] [PubMed]
- Beyar R, Goldstein Y. Model studies of the effects of the thoracic pressure on the circulation. Ann Biomed Eng 1987;15:373-83. [Crossref] [PubMed]
- Shuey CB Jr, Pierce AK, Johnson RL Jr. An evaluation of exercise tests in chronic obstructive lung disease. J Appl Physiol 1969;27:256-61. [Crossref] [PubMed]
- Pinsky MR, Desmet JM, Vincent JL. Effect of positive end-expiratory pressure on right ventricular function in humans. Am Rev Respir Dis 1992;146:681-7. [Crossref] [PubMed]
- Morimont P, Lambermont B, Ghuysen A, et al. Effective arterial elastance as an index of pulmonary vascular load. Am J Physiol Heart Circ Physiol 2008;294:H2736-42. [Crossref] [PubMed]
- Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev 2008;4:49-59. [Crossref] [PubMed]
- Vieillard-Baron A, Loubieres Y, Schmitt JM, et al. Cyclic changes in right ventricular output impedance during mechanical ventilation. J Appl Physiol (1985) 1999;87:1644-50. [Crossref] [PubMed]
- West JB. Understanding pulmonary gas exchange: ventilation-perfusion relationships. J Appl Physiol (1985) 2004;97:1603-4. [Crossref] [PubMed]
- Jardin F, Genevray B, Brun-Ney D, et al. Influence of lung and chest wall compliances on transmission of airway pressure to the pleural space in critically ill patients. Chest 1985;88:653-8. [Crossref] [PubMed]
- Magder S. The left heart can only be as good as the right heart: determinants of function and dysfunction of the right ventricle. Crit Care Resusc 2007;9:344-51. [PubMed]
- Voorhees AP, Han HC. Biomechanics of Cardiac Function. Compr Physiol 2015;5:1623-44. [Crossref] [PubMed]
- Buckberg G, Hoffman JI. Right ventricular architecture responsible for mechanical performance: unifying role of ventricular septum. J Thorac Cardiovasc Surg 2014;148:3166-71.e1-4.
- Yamaguchi S, Harasawa H, Li KS, et al. Comparative significance in systolic ventricular interaction. Cardiovasc Res 1991;25:774-83. [Crossref] [PubMed]
- Taylor RR, Covell JW, Sonnenblick EH, et al. Dependence of ventricular distensibility on filling of the opposite ventricle. Am J Physiol 1967;213:711-8. [Crossref] [PubMed]
- Magder S, Guerard B. Heart-lung interactions and pulmonary buffering: lessons from a computational modeling study. Respir Physiol Neurobiol 2012;182:60-70. [Crossref] [PubMed]
- Pinsky MR, Matuschak GM, Bernardi L, et al. Hemodynamic effects of cardiac cycle-specific increases in intrathoracic pressure. J Appl Physiol (1985) 1986;60:604-12. [Crossref] [PubMed]
- Lessard MR, Guerot E, Lorino H, et al. Effects of pressure-controlled with different I:E ratios versus volume-controlled ventilation on respiratory mechanics, gas exchange, and hemodynamics in patients with adult respiratory distress syndrome. Anesthesiology 1994;80:983-91. [Crossref] [PubMed]
- Chan K, Abraham E. Effects of inverse ratio ventilation on cardiorespiratory parameters in severe respiratory failure. Chest 1992;102:1556-61. [Crossref] [PubMed]
- Dries DJ, Kumar P, Mathru M, et al. Hemodynamic effects of pressure support ventilation in cardiac surgery patients. Am Surg 1991;57:122-5. [PubMed]
- Abraham E, Yoshihara G. Cardiorespiratory effects of pressure controlled ventilation in severe respiratory failure. Chest 1990;98:1445-9. [Crossref] [PubMed]
- Poelaert JI, Visser CA, Everaert JA, et al. Acute hemodynamic changes of pressure-controlled inverse ratio ventilation in the adult respiratory distress syndrome. A transesophageal echocardiographic and Doppler study. Chest 1993;104:214-9. [Crossref] [PubMed]
- Davis K Jr, Branson RD, Campbell RS, et al. Comparison of volume control and pressure control ventilation: is flow waveform the difference? J Trauma 1996;41:808-14. [Crossref] [PubMed]
- Singer M, Vermaat J, Hall G, et al. Hemodynamic effects of manual hyperinflation in critically ill mechanically ventilated patients. Chest 1994;106:1182-7. [Crossref] [PubMed]


