Paediatric gliomas: diagnosis, molecular biology and management
Introduction
Gliomas are brain tumours derived from glial cells, which are responsible for neuron cell support. The incidence of brain tumours in children lies at 5 cases per 100,000 population, 75% of which are classified as gliomas (1). The latter represents the most common category of brain tumours and pose a great neurosurgical challenge. In order to maximize patient survival, aggressive tumour removal is required, and “GTR” (gross total resection) should be the optimal result of every operation (2), especially for high grade gliomas (HGG). Subsequently, since histological types of paediatric gliomas favour lower grade lesions, there is a much higher chance of survival through effective management than adults (3).
Gliomas can be divided into three distinct general categories. HGG, low grade gliomas (LGG) and the separate category of diffuse intrinsic pontine gliomas (DIPG). The latter although officially part of HGG should be viewed separately due to their specific nature, molecular characteristics and management strategy. These three tumour entities require different treatment strategies. LGG can be followed conservatively and not operated although there is much controversy throughout the literature on this course of action. The general consensus is to operate paediatric patients with suspected LGG, but many authors suggest a conservative course of action for asymptomatic stable lesions with imaging characteristics of LGG (4). HGG must always be operated unless there are specific contradictions such as very critical tumour location, butterfly appearance, or extended lesions covering the dominant hemisphere. DIPG are considered inoperable and are managed with chemoradiotherapy. It is widely accepted that for most cases of HGG an adjunctive therapeutic strategy of radiotherapy and/or chemotherapy is the optimal treatment. However, evidence is lacking for an adjunctive treatment plan for LGG. The most accepted management strategy is to utilize adjunctive chemotherapy in cases of incomplete LGG resection (2). Finally, radiosurgery is considered to be an option not only for inoperable cases of HGG but also for cases of glioma recurrences and residual tumours.
Pathology
Histological features
Paediatric gliomas are classified according to histological criteria into a grading system of malignancy by World Health Organisation (WHO) (5). WHO grades I–IV have extremely different 5-year survival rates up from 95% for grades I and II gliomas and down to less than 10% for grade IV gliomas or even less than 1% for DIPG. We should also note that a 30–40% rise has been reported recently in median survival of patients with grades II and III gliomas, reaching the level of 2–3 years (6). Nevertheless, it must be noted that even among two tumours with identical histological grade, there can be variations in survival taking into account genetic markers, and specific genes affecting histological grading which will be discussed later on. Gliomas are derived from glial cells that have the potential to evolve into LGG, such as pilocytic astrocytoma, ganglioglioma, dysembryoplastic neuroepithelial tumour (DNET), diffuse glioma, HGG such as anaplastic astrocytoma, glioblastoma multiforme, as well as other types of gliomas such as ependymomas, oligodendrogliomas, brain stem gliomas, optic nerve gliomas, and finally mixed type gliomas (1). Even though tumour cell origin does not directly play a role in clinical course and treatment since histological grade alone dictates management course, cell origin-based subtypes usually have specific histological grade distribution. Therefore, ependymomas, for example, do not stand above grade III, brain stem gliomas are classified more often as grade IV, whilst optic nerve gliomas tend to be of lower histological grade, and glioblastoma multiforme is always classified as grade IV, etc. About two-thirds of total gliomas are low grade and thus of better prognosis, and one third are considered as high grade (7).
Genetics
Genetic changes have been extensively investigated in the recent years, and provide significant information associated with glioma prognosis and clinical course. According to recent data, genetics have been proven predictive of tumour behaviour and they offer significant insight in therapeutic decisions. They have not yet been directly incorporated in glioma management decision algorithms, however due to their correlation to tumour’s clinical behaviour they might play role in future therapeutic protocols and especially when deciding adjuvant medical treatment regimens, radiotherapy or radiosurgery approaches. Although not yet directly affecting management, genes have been incorporated in histological grading protocols. The presence of IDH 1/2 (isocitrate dehydrogenase 1) and p.K27M mutations in HIST1H3B genes, can move an otherwise lower grade tumour to a Grade IV classification and indirectly affect management (5). A list of the most central genes for paediatric gliomas is presented in Table 1 indicating the significance of each gene and possible management implications. Histone gene mutations are much more common in children and young adults, and this is the main genetic difference between adult and paediatric HGGs. H3F3A histone gene mutations have been linked to a much lower survival (13 months) for malignant brainstem gliomas (8). H3G34 mutant tumours are considered by many authors as a separate nosologic entity that should be separated from conventional histological grading classification (9). Another histone, HIST1H3B and the p.K27M substitution have been studied in the majority of DIPGs. IDH 1/2 mutations also play a pivotal role in glioma malignancy and dedifferentiation, being commonly detected in adult LGGs, and not in the paediatric population (10). Other mutations associated with HGG include ATRX, ALK2 gene mutations and NTRK gene fusions (11). BRAF (v-Raf murine sarcoma viral oncogene homolog B) mutations and fusions are very often encountered in low grade paediatric gliomas (10). Notable fusions which however have not yet been linked with prognosis and require further study include FXR1-BRAF, BRAF-MACF1. From BRAF gene mutations, BRAF V600E is the most common and has been linked with lower survival. Other genes which seem to be involved in paediatric LGGs are NF1, H3F3A, FGFR1 (fibroblast growth factor receptor 1). Infiltrative diffuse astrocytoma’s (grade III), frequently harbour MYB and MYBL1 (Myb proto-oncogene protein) gene rearrangements that play a role in infiltrative lower grade glioma behaviour. TP53 mutations are also commonly detected in diffuse astrocytoma’s, indicating infiltrative tumour behaviour (12). Furthermore, other genes studied in gliomas, such as MGMT (O-6-methylguanine-DNA methyltransferase) methylation or TERT promoter (Telomerase reverse transcriptase) mutations, are not commonly found in the paediatric population (10,13).

Full table
Molecular biology
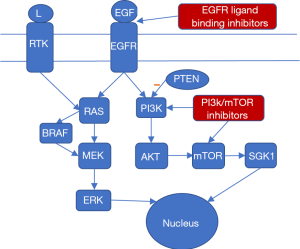
In order to identify the genes affecting glioma prognosis and response to treatment, it is imperative to study the molecular biology which powers glioma proliferation. The paediatric population naturally has the same molecular biology as the adult population, with very few differences, such as the relative absence of IDH mutations in paediatric LGGs and the much higher prevalence of histone gene mutations. However paediatric gliomas are commonly of lower grade and therefore low-grade glioma pathways are much more meaningful when studying paediatric gliomas. Molecular biology data are of great importance since they can guide molecular therapy, which is currently considered as the future of cancer treatment (14). However, it is true that molecular glioma treatment is currently at a very nascent stage and no actual clinical application as yet. The main pathways affected in HGG according to the literature are the EGF (epidermal growth factor) and VEGF (vascular endothelial growth factor) pathways which are responsible for angiogenesis. Angiogenesis plays an important role in tumour growth and diffusive malignant behaviour. PDGFR (platelet-derived growth factor receptors) and EGFR (epidermal growth factor receptor), parts of the PI3K (phosphatidylinositol-4,5-bisphosphate 3-kinase) signalling cascade, play a role in cell proliferation and also seem to be important in glioma development. Another important molecular pathway in HGG is the deregulated p53/RB (tumor protein 53, retinoblastoma protein) pathway, which seems to be affected in over 80% of HGG cases. Mutations in this pathway have been associated with malignant high-grade tumor behavior. LGG present only a few molecular differences. The most studied pathway is the MAP (mitogen-activated protein) kinase pathway, and more specifically BRAF and FGFR1 kinases. The key difference between high grade and low-grade glioma molecular biology lies on the fact that HGGs are linked with dysfunction in pathways involved in angiogenesis, which is one of the main characteristics of malignant neoplasms in general. However, lower grade gliomas involve mainly defects on cell division cycle pathways, such as the MAP kinase pathway, which represents the first molecular step in tumor evolution (14). Finally, another important molecular pathway affected in pediatric gliomas which involves both HGG and LGGs is the PI3 Kinase (PI3K/mTOR) pathway (15). Loss of function of PTEN (phosphatase and tensin homolog) which is the main negative PI3K regulator or PI3K overactivation can lead to malignant tumor behavior (11). The aforementioned pathways present the basis for tailoring of a more targeted molecular treatment with MAPK pathway being commonly targeted with BRAF or MEK inhibitors (16). EGFR pathway is also a very popular molecular treatment pathway usually through monoclonal antibodies that disrupt EGFR ligand binding (17). Clinical trial studies show that dual PI3K/mTOR inhibitors are promising therapeutic agents for future pediatric glioma treatment (15). The best studied molecular pathways in pediatric gliomas and their interconnections are shown schematically in Figure 1 along with potential molecular treatment options.
Clinical diagnosis
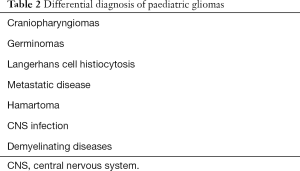
Paediatric tumours can remain undiagnosed for a prolonged period of time. Patients in these age groups are not capable to effectively present their symptoms, and some common symptoms which might be non-specific such as headaches can easily be missed (13). Although the majority of LGGs (over 80%) will present through episodes of seizures facilitating diagnosis, there is a sufficient percentage of asymptomatic patients (18) that contribute to a lag in diagnosis and allow further glioma growth. Therefore, an improvement in paediatric glioma diagnosis is highly needed. It is important for clinicians to be very thorough when evaluating paediatric patients with neurological symptoms, which give rise to suspicion for central nervous system (CNS) pathology (13). The presentation may include a headache, changes in behaviour, diplopia, emesis and nausea, as well as papilledema. Other more specific symptoms are focal motor deficits such as lesions of the pyramidal tract, hemiplegia, chorea and dysmetria. Another presentation is through episodes of seizures which are frequent in cases of LGG, and much scarcer in HGGs. The differential diagnosis for gliomas is quite extensive, including CNS infections, such as viral encephalitis, primary epilepsy and the very wide array of paediatric demyelinating CNS syndromes (19). A list of the main diseases to consider for differential diagnosis of paediatric glioma cases is given in Table 2 (1). Clinical examination is not adequate for glioma diagnosis. Although it is possible to hypothesize lesion location, it is not possible to differentiate the nature of the lesion (20). This question must be addressed through imaging, or through stereotactic or intraoperative biopsy. Paediatric glioma patients also show a high prevalence of genetic predisposition syndromes, such as neurofibromatosis I or tuberous sclerosis. These special patient categories are commonly affected by multiple tumours, have a worse prognosis, and are very challenging for clinicians and surgeons (21).
Full table
Imaging
The main diagnostic modality for gliomas and tumours, in general is imaging which mainly includes computed tomography (CT) and magnetic resonance imaging (MRI), and also newer imaging techniques such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET). CT is useful to detect erosion of bony structures as well as hemorrhagic tumour attributes and also offers a fast method for initial diagnosis (1). Novel imaging technologies offer improved knowledge of tumor nature and anatomy, indicate its relationship with adjacent critical structures and increase diagnostic accuracy, but there is a lack of studies in the literature linking their use with better clinical outcomes. Diagnostic accuracy through imaging is generally satisfying especially for LGGs approaching the level of 90%. A slightly higher level has been reported for more advanced techniques such as PET scan and advanced MRI-based techniques such as diffusion MRI, or MRI spectroscopy (22). Advanced imaging techniques have the potential to greatly affect pediatric cases of gliomas. Due to the increased life expectancy in the pediatric population, even a minor difference in diagnostic accuracy or treatment efficacy can be translated in a substantial survival gain. In addition, advanced imaging techniques are proved to slightly improve surgical excision and patients’ survival (23). These concepts could justify the use of expensive techniques such as PET scan in the pediatric population with suspected brain gliomas (19). However evidence on diagnostic accuracy is scarce, and more research is needed to draw definite conclusions (19,22,24). The lack of clinical trials, the lack of wide scale availability and the relatively high cost of such techniques are the main reasons of their underutilization, although there is some evidence indicating cost-effectiveness of PET scan for glioma diagnosis (23,25,26). Currently the grade and quantity of evidence is insufficient to justify the wide-scale use of advanced imaging techniques for glioma diagnosis, and available positive evidence should be regarded with caution. Therefore, new perspective or randomized studies are much required. Imaging is not considered 100% diagnostic and a biopsy is required in most cases of gliomas (1). A lesion diagnosed solely through imaging which does not require a biopsy for definite diagnosis is the DIPG.
Treatment options
Chemotherapy
There are numerous regimens of chemotherapy which can be utilized for LGGs, HGGs and recurrent gliomas. Chemotherapy, when examined alone, seems to be the less effective modality, offering an only slight increase in survival (27). However, it is utilized due to its cumulative effect when combined with other management strategies, an effect that can provide dramatic increases in survival, up to three-fold (28). CCNU (chloroethyl-cyclohexyl nitrosourea) and vincristine are some of the main chemotherapeutic agents utilized with great effect in various clinical trials, as well as PCV (procarbazine, lomustine, and vincristine) which has also been reported to improve survival in cases of paediatric gliomas. Temozolomide (TMZ) is a standard chemotherapeutic modality which appears to increase median survival for approximately 2 months in adults, but trials in children failed to show any survival benefit (1). Chemotherapy with the transplantation of stem cells is a novel modality for treating HGG, although its superiority to other treatment modalities has not been established (29).
Radiotherapy
Radiotherapy is a very effective treatment modality for gliomas, especially for LGGs. It can be utilized either post-operatively or preoperatively, or as a stand-alone treatment for inoperable cases (30). It is most often combined with chemotherapy a combination which seems to increase toxicity. Recent studies have pinpointed that combination with temozolomide produces less toxicity than the combination with cisplatinum-based regimens (31). Radiotherapy can also be augmented by the use of radiosensitizers such as gemcitabine, improving patient survival and increasing quality of life (QoL) (32). Radiotherapy is utilized in glioma management after tumour resection in virtually all cases. It is also used in inoperable cases along with radiosurgery and radiotherapy. The usual dose is about 60 Gray (Gy) in 30 fractions while higher doses do not seem to affect survival (1).
Radiosurgery
Radiosurgery in the management of pediatric gliomas is controversial and there is no consensus on its utilization. The main concerns are collateral tissue damage and devastating brain edema that can both negatively affect prognosis and/or create great difficulties for future surgical resection (33). However, gamma knife surgery has proved most effective in cases of inoperable LGGs, HGGs and glioma recurrences offering satisfying tumour control, especially in cases of residual tumours of small volume (34). Studies show that radiosurgery decreases residual tumour volume up to 70% and higher residual volumes tend to respond less to gamma knife surgery. Exact tumour volume limits have not been determined but based on the literature, volumes of 2 cm3 or less tend to respond well to radiosurgery and tumour volumes of 4 cm3 and above tend to respond poorly (33). The usual radiation dosage on the tumour margin is approximately 15 Gy (35).
Neurosurgical management
The ultimate treatment of gliomas has always been complete surgical excision, a strategy with great technical difficulties. Both LGG and HGG should be operated although there are suggestions of a conservative follow up of some selected LGG cases with serial brain imaging studies. However, recent studies have shown that follow up of LGGs is not as effective as the standard surgical management protocols (2). Therefore, surgery should be the initial management step of every glioma case regardless of grade except of some specific glioma types with contradictions such as DIPG. Neurosurgical treatment should be aggressive and throughout in all glioma cases. It has been proven that GTR offers a substantial advantage in survival over subtotal resection. Recent studies have shown that subtotal resection increases mortality as much as infratentorial tumour location (36), which translates to a 50–100% increased mortality for patients with subtotal resection (36,37). In terms of months duration, the difference in survival can reach up to 35 months when comparing GTR to subtotal resection (37). According to literature, GTR is the most important feature in the treatment of gliomas, and therefore the role of the neurosurgeon is of pivotal importance. It must be noted that in adult patients the difference in survival is much lower (30%) and is mainly attributed to the higher life expectancy of the paediatric population (38). For the above reasons, neurosurgical treatment of paediatric gliomas must be more aggressive (3). GTR, however, is not easily achieved. Studies show that only 35–45% of paediatric patients receive GTR (37,39). This pinpoints the need to increase surgical precision through new technologies, but studies showing increased GTR percentages when utilizing newer technologies such as intraoperative MRI are scarce. Currently, some studies show that intraoperative imaging through fluorescence or MRI can increase GTR rates, but the amount and quality of evidence are not sufficient to draw definite conclusions (40,41). The most common factor affecting GTR percentages is superficial tumour location. GTR seems to be mostly feasible in superficial tumors and rates are much lower for deep-seated brain tumors (37). Over the last decades, studies show that survival in GBM paediatric patients has not been substantially improved. However, survival of patients not receiving GTR has been improved though the use of radiotherapy (42). Radiation and chemotherapy have been reported to improve the survival of patients where GTR cannot be achieved. Since GTR is the most powerful predictor of glioma survival, the fact that survival has not increased in the past decades indicates the need to improve GTR rates in paediatric gliomas (43).
Palliative treatment
Palliative and end of life care are very important since many glioma cases are inoperable, due to contradictions or recurrent disease. Therefore, in many cases, palliative care is the only option a physician can offer. Palliative care consists of family counselling and minor interventions, which slightly increase quality of life for the remaining months of life. Prognosis of inoperable cases such as DIPG can be extremely low with a 5-year survival of less than 1% (44), and an average of 12 months remaining life (45). Although such a time period seems limited, it is a rather critical stressful time with both patients and families requiring both physical and psychological care. Palliative care includes adequate family counseling, psychological support, supportive treatment, and various minor interventions such as the installation of external ventricular drainage systems for the alleviation of hydrocephalus symptoms (46). The most important part of pediatric palliative care is the psychological support which is a very delicate matter for patients and families (47). Studies show that during DIPG clinical course, the most affected quality of life aspects are anxiety and physical functioning (48). Although palliative treatment is very important for inoperable gliomas, there are no protocols guiding effective palliative care (49). It is generally a procedure decided and planned by surgeons and oncologists individually and many physicians, unfortunately, neglect some important aspects of palliative care such as the psychological support (50). A recent cohort study has identified the most widely used palliative interventions. Analgesia, antiemetics, steroids, anticonvulsants, anti-secretory drugs, and laxatives are all measures utilized in more than 50% of glioma patients (50).
Discussion
Management of paediatric gliomas can be either through palliative care, conservative treatment, chemotherapy, radiotherapy, surgical management or a combination of the above. Treatment depends on the type of glioma. Palliative care includes end of life treatment in cases of gliomas that are not amenable to resection or non-surgical treatment. Large grade IV gliomas involving the dominant hemisphere and/or extending to the contralateral hemisphere (butterfly gliomas) are some very common examples (2,28,51). It is, of course, possible to perform whole brain radiation, hypo fractionated radiotherapy, and surgical excision of remnants, but such strategies do not offer additional survival benefit (52). Palliative care includes supportive care such as brain oedema management with osmotic agents and/or corticosteroids, sufficient pain relief and family counselling. Conservative management of gliomas also includes corticosteroids for the alleviation of brain oedema, prophylactic use of anticonvulsants, and in some cases careful waiting until surgery or other management strategies. LGGs are preferably excised, but an alternative is watchful waiting for LGGs of small size in non-eloquent areas. Many authors support that watchful waiting might be inferior to early resection for LGGs, although the amount and quality of evidence is insufficient (2). We must point out that especially for the paediatric population the majority of the authors repost that conservative treatment is a less favourable option (2,4,53). It is mostly preferable to follow an aggressive surgical approach, since children have a higher life expectancy, and aggressive treatment can offer increased clinical benefit (54). Utilizing a conservative approach should be reserved for special cases with significant comorbidities and/or specific contradictions. In addition, conservative management in such paediatric cases of gliomas should be accompanied with a closer follow up (43) during which a surgical approach should be engaged when and if indicated.
HGG, on the other hand, are always managed aggressively unless specific contradictions exist, such as butterfly gliomas, multicentre gliomas, extensive dominant lobe involvement, or poor prognosis such as elderly patients or low Karnofsky score (<70) (13). Surgery with the removal of the maximum possible percentage and GTR should always be the goal. Surgery is always followed by chemotherapy (temozolomide) and radiotherapy (external beam radiation) for optimal results. The special case of DIPG is considered inoperable and managed through chemotherapy and radiotherapy, but with poor results (9-month median survival) (50,55). However, there are some promising new studies on the oncolytic virus DNX-2401, as a possible future treatment modality for such extremely malignant cases (56).
Recurrences are similarly if excision is feasible. Radiosurgery is also an option for recurrences and is very effective in cases of low tumour volumes. Therefore, when managing recurrences of paediatric gliomas, the decision is between surgical excision with chemoradiotherapy when surgical excision is possible, radiosurgery with chemoradiotherapy when tumour location is non-critical, and tumour volume is sufficiently low, and chemoradiotherapy alone when tumour location and/or size precludes the use of surgery and radiosurgery (57). Inoperable cases can be managed with radiosurgery, if collateral damage is acceptable, and subsequent chemotherapy and radiotherapy. When radiosurgery is not acceptable, then only chemotherapy and radiotherapy should be utilized (1).
Paediatric gliomas differ from adult gliomas, in terms of differentiation and biology (58). The paediatric population is mostly affected by lower grade gliomas and the affected pathways usually involve mitotic and cell division mechanisms. In addition, paediatric patients receive a much higher survival benefit from interventions than adults, which highlights the significance of even slightly superior interventions (36). This also increases the value of aggressive treatment, and the use of advanced imaging technologies and intraoperative imaging. There is a great need for high-quality studies to assess the value of novel technologies on the management of paediatric gliomas. Such research would have a high impact on patient survival and quality of life (3).
Early diagnosis is extremely important, in order to proceed earlier with tumour management and achieve higher rates of GTR. When it comes to paediatric patient evaluation for possible gliomas diagnostic protocols should be more aggressive. The quality-adjusted life years (QALYs) saved through aggressive diagnostic protocols, can justify the additional cost (1). The most important goal is to improve GTR rates through new approaches and technologies, and there is a great need for additional studies and research in this area. It is also important to better study glioma biology in order to develop novel treatment modalities and facilitate management and surgical resection. Research is also needed in all other areas of glioma such as chemotherapy, radiotherapy and radiosurgery in order to improve patients’ survival and QALYs. Only by focusing on all treatment modalities along with effective surgical intervention it will be possible to maximize median survival. The future of paediatric glioma treatment might reveal an unexpected increase in survival, due to increasing scientific research data and technological development (59).
Conclusions
Paediatric gliomas are very diverse and prognosis mainly depends on their histological grade. Tumours located in critical areas such as infratentorial location and brain stem and deep-seated gliomas are much harder to excise and carry a worse prognosis. There has been great interest in the molecular genetics of gliomas. However, this great amount of research has not lead to increased survival. During the recent decades, survival of patients with gliomas has not been substantially increased, a fact which pinpoints the need for additional research on more critical areas affecting survival. There is hope that novel molecular treatment options will become available through the research of the molecular pathways which affect glioma differentiation and proliferation. Molecular personalized treatment might be the main therapeutic approach of gliomas in the future, bypassing the need for other treatment strategies. Clinical diagnosis and imaging of gliomas hold a high overall diagnostic accuracy surpassing 90%. However, the early diagnosis of gliomas can improve survival and therefore there is a need for improvement, possibly through advanced imaging technologies or through screening protocols. There are multiple treatment modalities, but the universally accepted strategy consists of the triad of tumour resection, radiotherapy and chemotherapy. Tumour recurrences and inoperable tumours are usually managed through radiosurgery and further sessions of radiotherapy and chemotherapy. The most important part of glioma treatment and the one affecting survival more than any other metric is the extent of tumour resection. GTR when compared to subtotal resection can triple patient’s survival by months and therefore new technologies should be developed to facilitate GTR in order to improve patient’s prognosis.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Diwanji TP, Engelman A, Snider JW, et al. Epidemiology, diagnosis, and optimal management of glioma in adolescents and young adults. Adolesc Health Med Ther 2017;8:99-113. [Crossref] [PubMed]
- van den Bent MJ, Snijders TJ, Bromberg JE. Current treatment of low grade gliomas. Memo 2012;5:223-7. [Crossref] [PubMed]
- Bilginer B, Hanalioglu S, Turk CC, et al. Is the knowledge pertained to adult glioblastomas enough for pediatric cases? prognostic factors in childhood. Turk Neurosurg 2017;27:279-88. [PubMed]
- Ali ZS, Lang SS, Sutton LN. Conservative Management of Presumed Low-Grade Gliomas in the Asymptomatic Pediatric Population. World Neurosurg 2014;81:368-73. [Crossref] [PubMed]
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016;131:803-20. [Crossref] [PubMed]
- Dong X, Noorbakhsh A, Hirshman BR, et al. Survival trends of grade I, II, and III astrocytoma patients and associated clinical practice patterns between 1999 and 2010: A SEER-based analysis. Neuro Oncol Pract 2015;3. [Crossref]
- Qaddoumi I, Sultan I, Gajjar A. Outcome and prognostic features in pediatric gliomas. Cancer 2009;115:5761-70. [Crossref] [PubMed]
- Zhang Y, Pan C, Wang J, et al. Genetic and immune features of resectable malignant brainstem gliomas. Oncotarget 2017;8:82571-82. [PubMed]
- Korshunov A, Capper D, Reuss D, et al. Histologically distinct neuroepithelial tumors with histone 3 G34 mutation are molecularly similar and comprise a single nosologic entity. Acta Neuropathol 2016;131:137-46. [Crossref] [PubMed]
- Venneti S, Huse JT. The Evolving Molecular Genetics of Low-grade Glioma. Adv Anat Pathol 2015;22:94-101. [Crossref] [PubMed]
- Diaz AK, Baker SJ. The genetic signatures of pediatric high-grade glioma: no longer a one-act play. Semin Radiat Oncol 2014;24:240-7. [Crossref] [PubMed]
- Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 2013;45:602-12. [Crossref] [PubMed]
- Fangusaro J. Pediatric high grade glioma: a review and update on tumor clinical characteristics and biology. Front Oncol 2012;2:105. [Crossref] [PubMed]
- Meel MH, Schaper SA, Kaspers GJ, et al. Signaling pathways and mesenchymal transition in pediatric high-grade glioma. Cell Mol Life Sci 2018;75:871-87. [Crossref] [PubMed]
- Dasgupta T, Haas-Kogan DA. The Combination of Novel Targeted Molecular Agents and Radiation in the Treatment of Pediatric Gliomas. Front Oncol 2013;3:110. [Crossref] [PubMed]
- Bavle A, Jones J, Lin FY, et al. Dramatic clinical and radiographic response to BRAF inhibition in a patient with progressive disseminated optic pathway glioma refractory to MEK inhibition. Pediatr Hematol Oncol 2017;34:254-9. [Crossref] [PubMed]
- Taylor TE, Furnari FB, Cavenee WK. Targeting EGFR for treatment of glioblastoma: molecular basis to overcome resistance. Curr Cancer Drug Targets 2012;12:197-209. [Crossref] [PubMed]
- Rudà R, Bello L, Duffau H, et al. Seizures in low-grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol 2012;14 Suppl 4:iv55-64. [Crossref] [PubMed]
- Kazda T, Bulik M, Pospisil P, et al. Advanced MRI increases the diagnostic accuracy of recurrent glioblastoma: Single institution thresholds and validation of MR spectroscopy and diffusion weighted MR imaging. NeuroImage Clin 2016;11:316-21. [Crossref] [PubMed]
- Ehrstedt C, Kristiansen I, Ahlsten G, et al. Clinical characteristics and late effects in CNS tumours of childhood: Do not forget long term follow-up of the low grade tumours. Eur J Paediatr Neurol 2016;20:580-7. [Crossref] [PubMed]
- Guerreiro Stucklin AS, Tabori U, Grotzer M. The Changing Landscape of Pediatric Low-Grade Gliomas: Clinical Challenges and Emerging Therapies. Neuropediatrics 2016;47:70-83. [Crossref] [PubMed]
- Verburg N, Hoefnagels FW, Barkhof F, et al. Diagnostic Accuracy of Neuroimaging to Delineate Diffuse Gliomas within the Brain: A Meta-Analysis. AJNR Am J Neuroradiol 2017;38:1884-91. [Crossref] [PubMed]
- Fischer BM, Siegel BA, Weber WA, et al. PET/CT is a cost-effective tool against cancer: synergy supersedes singularity. Eur J Nucl Med Mol Imaging 2016;43:1749-52. [Crossref] [PubMed]
- Nihashi T, Dahabreh IJ, Terasawa T. Diagnostic Accuracy of PET for Recurrent Glioma Diagnosis: A Meta-Analysis. Am J Neuroradiol 2013;34:944-50. [Crossref] [PubMed]
- Heinzel A, Stock S, Langen KJ, et al. Cost-effectiveness analysis of amino acid PET-guided surgery for supratentorial high-grade gliomas. J Nucl Med 2012;53:552-8. [Crossref] [PubMed]
- Heinzel A, Stock S, Langen KJ, et al. Cost-effectiveness analysis of FET PET-guided target selection for the diagnosis of gliomas. Eur J Nucl Med Mol Imaging 2012;39:1089-96. [Crossref] [PubMed]
- Kline C, Felton E, Allen IE, et al. Survival outcomes in pediatric recurrent high-grade glioma: results of a 20-year systematic review and meta-analysis. J Neurooncol 2018;137:103-10. [Crossref] [PubMed]
- El-Ayadi M, Ansari M, Sturm D, et al. High-grade glioma in very young children: a rare and particular patient population. Oncotarget 2017;8:64564-78. [Crossref] [PubMed]
- Durando X, Lemaire JJ, Tortochaux J, et al. High-dose BCNU followed by autologous hematopoietic stem cell transplantation in supratentorial high-grade malignant gliomas: a retrospective analysis of 114 patients. Bone Marrow Transplant 2003;31:559-64. [Crossref] [PubMed]
- Mak KS, Lee SW, Balboni TA, et al. Clinical outcomes and toxicity following palliative radiotherapy for childhood cancers. Pediatr Blood Cancer 2018;65. [Crossref] [PubMed]
- Seidel C, von Bueren AO, Bojko S, et al. Concurrent radiotherapy with temozolomide vs. concurrent radiotherapy with a cisplatinum-based polychemotherapy regimen. Strahlenther Onkol 2018;194:215-24. [Crossref] [PubMed]
- Veldhuijzen van Zanten SEM, El-Khouly FE, Jansen MHA, et al. A phase I/II study of gemcitabine during radiotherapy in children with newly diagnosed diffuse intrinsic pontine glioma. J Neurooncol 2017;135:307-15. [Crossref] [PubMed]
- Weintraub D, Yen CP, Xu Z, et al. Gamma Knife surgery of pediatric gliomas. J Neurosurg Pediatr 2012;10:471-7. [Crossref] [PubMed]
- Kano H, Niranjan A, Kondziolka D, et al. Stereotactic radiosurgery for pilocytic astrocytomas part 2: outcomes in pediatric patients. J Neurooncol 2009;95:219-29. [Crossref] [PubMed]
- Kano H, Yang H, Kondziolka D, et al. Stereotactic radiosurgery for pediatric recurrent intracranial ependymomas. J Neurosurg Pediatr 2010;6:417-23. [Crossref] [PubMed]
- Lam S, Lin Y, Zinn P, et al. Patient and treatment factors associated with survival among pediatric glioblastoma patients: A Surveillance, Epidemiology, and End Results study. J Clin Neurosci 2018;47:285-93. [Crossref] [PubMed]
- Yang T, Temkin N, Barber J, et al. Gross Total Resection Correlates with Long-Term Survival in Pediatric Patients with Glioblastoma. World Neurosurg 2013;79:537-44. [Crossref] [PubMed]
- Brown TJ, Brennan MC, Li M, et al. Association of the Extent of Resection With Survival in Glioblastoma. JAMA Oncol 2016;2:1460. [Crossref] [PubMed]
- Adams H, Adams HH, Jackson C, et al. Evaluating extent of resection in pediatric glioblastoma: a multiple propensity score-adjusted population-based analysis. Childs Nerv Syst 2016;32:493-503. [Crossref] [PubMed]
- Bradley WG. Achieving gross total resection of brain tumors: intraoperative MR imaging can make a big difference. AJNR Am J Neuroradiol 2002;23:348-9. [PubMed]
- Chen B, Wang H, Ge P, et al. Gross total resection of glioma with the intraoperative fluorescence-guidance of fluorescein sodium. Int J Med Sci 2012;9:708-14. [Crossref] [PubMed]
- Chamdine O, Gajjar A. Molecular characteristics of pediatric high-grade gliomas. CNS Oncol 2014;3:433-43. [Crossref] [PubMed]
- Youland RS, Khwaja SS, Schomas DA, et al. Prognostic Factors and Survival Patterns in Pediatric Low-grade Gliomas Over 4 Decades. J Pediatr Hematol Oncol 2013;35:197-205. [Crossref] [PubMed]
- Korones DN. Treatment of newly diagnosed diffuse brain stem gliomas in children: in search of the holy grail. Expert Rev Anticancer Ther 2007;7:663-74. [Crossref] [PubMed]
- Janssens GO, Gandola L, Bolle S, et al. Survival benefit for patients with diffuse intrinsic pontine glioma (DIPG) undergoing re-irradiation at first progression: A matched-cohort analysis on behalf of the SIOP-E-HGG/DIPG working group. Eur J Cancer 2017;73:38-47. [Crossref] [PubMed]
- Emelifeonwu JA, Sokol D, Gallo P, et al. Long-tunnelled external ventricular drain as a long-term treatment option for hydrocephalus in a child with an unresectable low-grade supratentorial tumor: case report. J Neurosurg Pediatr 2016;18:430-3. [Crossref] [PubMed]
- Marcus J. Psychosocial issues in pediatric oncology. Ochsner J 2012;12:211-5. [PubMed]
- Mandrell BN, Baker J, Levine D, et al. Children with minimal chance for cure: parent proxy of the child’s health-related quality of life and the effect on parental physical and mental health during treatment. J Neurooncol 2016;129:373-81. [Crossref] [PubMed]
- Fischer C, Petriccione M, Donzelli M, et al. Improving Care in Pediatric Neuro-oncology Patients: An Overview of the Unique Needs of Children With Brain Tumors. J Child Neurol 2016;31:488-505. [Crossref] [PubMed]
- Veldhuijzen van Zanten SE, van Meerwijk CL, Jansen MH, et al. Palliative and end-of-life care for children with diffuse intrinsic pontine glioma: results from a London cohort study and international survey. Neuro Oncol 2016;18:582-8. [Crossref] [PubMed]
- Pfister S, Witt O. Pediatric Gliomas. In: Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer 2009:67-81.
- Navarria P, Pessina F, Franzese C, et al. Hypofractionated radiation therapy (HFRT) versus conventional fractionated radiation therapy (CRT) for newly diagnosed glioblastoma patients. A propensity score matched analysis. Radiother Oncol 2018;127:108-13. [Crossref] [PubMed]
- Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol 2009;24:1397-408. [Crossref] [PubMed]
- Warren KE. Pediatric high-grade gliomas: survival at what cost? Transl Pediatr 2012;1:116-7. [PubMed]
- Gwak H-S, Park HJ. Developing chemotherapy for diffuse pontine intrinsic gliomas (DIPG). Crit Rev Oncol Hematol 2017;120:111-9. [Crossref] [PubMed]
- Tejada S, Alonso M, Patiño A, et al. Phase I Trial of DNX-2401 for Diffuse Intrinsic Pontine Glioma Newly Diagnosed in Pediatric Patients. Neurosurgery 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Mallick S, Benson R, Hakim A, Rath GK. Management of glioblastoma after recurrence: A changing paradigm. J Egypt Natl Canc Inst 2016;28:199-210. [Crossref] [PubMed]
- Rizzo D, Ruggiero A, Martini M, et al. Molecular Biology in Pediatric High-Grade Glioma: Impact on Prognosis and Treatment. Biomed Res Int 2015;2015. [Crossref] [PubMed]
- Broniscer A. Past, Present, and Future Strategies in the Treatment of High-Grade Glioma in Children. Cancer Invest 2006;24:77-81. [Crossref] [PubMed]


