Cervical or thoracic anastomosis for patients with cervicothoracic esophageal squamous cell carcinoma
Introduction
Esophageal cancer is a type of malignant tumor and has one of the highest mortality rates. Approximately 455,800 patients are newly diagnosed each year, and approximately 400,200 patients die of esophageal cancer each year (1). East Asia has the highest incidence of esophageal cancer worldwide, which is 21-times higher than that in West Africa, which has the lowest incidence. Moreover, more than 90% of patients in East Asia have esophageal squamous cell carcinoma. Surgery is the most effective treatment for esophageal cancer. A variety of surgical procedures are currently used in clinical practice, including the Ivor-Lewis, McKeown and Sweet procedures (2). The advantages and disadvantages of the various procedures are still contentious. However, right thoracic approaches, including the Ivor-Lewis procedure and the McKeown procedure, are considered to be more advantageous for the clearance of chest and abdominal lymph nodes, especially for the direct resection of bilateral recurrent laryngeal nerve (RLN) lymph nodes. Therefore, a right thoracic approach is considered to be a superior method (3).
Recently, the 8th edition of the TNM staging classification recommended the classification of esophageal tumors based on the length measured from the incisors to the epicenter of the esophageal tumor using an endoscope (4). According to this system, esophageal tumors can be classified as cervical (15–20 cm), upper thoracic (20–25 cm), middle thoracic (25–30 cm), lower thoracic (30–40 cm) and at the esophagogastric junction (40–42 cm). The upper edge of the sternal notch is considered an anatomical landmark of cervical and upper thoracic tumors, because the sternal notch represents the top of the thorax from the side view and is flush with T2 or T3. Therefore, the part of the cervical esophagus that is flush with T1–T3 is located in the thorax. If an esophageal tumor is flush with the upper edge of the sternal notch, a sufficient amount of cervical esophagus remains present in the thorax to enable an R0 resection and esophagogastric anastomosis without a neck incision; however, cervical lymph node resection is not possible (5). Therefore, we defined tumors that are flush with the sternal notch at 18–22 cm from the incisors as cervicothoracic tumors.
Japan is known to employ one of the most effective esophageal surgical techniques. Since 1983, Japanese surgeons have suggested that three-field (3FL) lymph node resection is the most effective treatment for esophageal tumors and can improve the prognosis of affected patients (6,7). However, the three-incision procedure is associated with extensive trauma, a longer operation time and higher mortality. Furthermore, the rate of postoperative complications, including anastomotic leakage, anastomotic stenosis, and RLN injury, is higher in cervical operations than in two-incision procedures (8-11). Shim et al. reported that among patients with thoracic esophageal tumors without cervical metastasis (radiological evaluations showing no cervical lymph nodes >1 cm), long-term survival between patients who underwent two-field (2FL) and 3FL resection was not significantly different (10). However, a lack of relevant research regarding cervicothoracic esophageal tumors has prevented verification of the long-term effects of thoracic anastomosis and cervical anastomosis. We conducted a single-center retrospective study to determine whether anastomosis in the neck or the thorax produced different perioperative results and long-term postoperative survival rates.
Methods
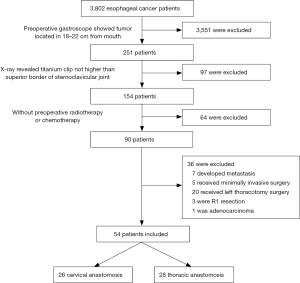
From January 2001 to January 2017, 3,802 consecutive patients with esophageal tumors were admitted to the Department of Cardiothoracic Surgery at Nanjing Drum Tower Hospital and underwent surgical treatment. All patients received preoperative gastroscopy. In the patients with tumors 18–22 cm inferior to the incisors on endoscopy, the upper edge of the tumor was marked with a titanium clip. Then, a chest X-ray was used to confirm the positional relationship between the titanium clip and the upper edge of the sternal notch. If the titanium clip was not higher than the upper edge of the sternal notch, thoracic anastomosis was considered. Otherwise, neck anastomosis was performed. The exclusion criteria were preoperative chemotherapy or radiotherapy; lymph node or distant metastases upon preoperative examination; minimally invasive surgery; a left surgical approach; R1 or R2 resection; or non-squamous cell carcinoma diagnosis upon postoperative pathological analysis (Figure 1). A total of 54 patients were enrolled in this study, including 26 with cervical anastomosis and 28 with thoracic anastomosis. All surgeries were performed by three experienced surgeons, and the choice of surgical approach was based on tumor location and intraoperative conditions. All TNM staging was performed according to the AJCC/UICC staging method. The study was approved by the institutional ethics board of Nanjing Drum Tower Hospital (2017-175-01).
Operative procedures
Cervical anastomosis
We performed the McKeown procedure for cervical anastomosis. Each patient was initially placed in the lateral position. A right posterolateral thoracotomy was performed at the fifth interspace. After esophageal tumor resection, mediastinal lymph nodes were dissected, including but not limited to paraesophageal nodes, bilateral RLN nodes, tracheobronchial nodes, posterior mediastinal nodes and superior mediastinal nodes. The thorax was closed after drainage tube placement, and then, the patient was placed in the supine position. A midline abdominal incision was performed, and the greater and lesser omentum was isolated to the pylorus. The right gastric-epiploic arterial arch was retained, and the left gastric artery and short gastric artery were divided. Celiac nodes, nodes along the left gastric artery, paracardiac nodes and common hepatic artery nodes were dissected. A gastric tube was created, and the vascular supply was provided by the right gastric and right gastric-epiploic arteries. The diaphragmatic hiatus was extended to pass the esophagus to the neck. Then, a jejunostomy was performed in all patients. A cervical incision was made parallel to the left sternocleidomastoid muscle. The gastric tube and esophagus were then divided, and the anastomosis was sutured. The lymph nodes below the level of the cricoid cartilage were dissected, including but not limited to cervical RLN chain nodes, internal jugular nodes, deep cervical nodes and cervical paraesophageal nodes. All patients with cervical anastomosis underwent 3FL resection.
Thoracic anastomosis
We performed the Ivor-Lewis procedure for thoracic anastomosis. The patients underwent abdominal surgery to create a gastric tube, and then, the anastomosis was sutured in the thorax by using an end-to-end anastomosis stapler. The other steps were the same as those used in the McKeown procedure, but these patients underwent 2FL resection. The gastric tube was placed in the original esophageal bed. During the operation, anesthesiologists adjusted the position of the nasogastric tube to maintain the end of the tube at 5 cm below the anastomosis. We routinely placed chest and mediastinal drainage tubes, but indwelling drainage tubes were not generally placed in the neck or abdomen.
Postoperative treatment
All patients were treated with intravenous and enteral nutrition for 6 days. After evaluating drainage fluid properties and performing X-rays using a swallowed contrast agent to ensure the absence of an anastomotic fistula, a liquid diet was initiated. After 1 day, the patients could ingest soft food. Finally, 10 days after surgery, the patients started a normal diet.
A postoperative anastomotic leak was determined when water-soluble contrast agent osmosis could be identified on X-ray or when clinical anastomotic fistula symptoms emerged. Postoperative anastomotic stenosis was determined when the contrast agent revealed stenosis on X-ray or when dysphagia was observed. RLN injury was indicated by the appearance of hoarseness or the occurrence of bucking when drinking or when diagnosed by fiber laryngoscopy.
Follow-up
All patients were followed up at 1, 3, 6 and 12 months postoperatively. All patients underwent computed tomography (CT) and/or gastroscopy to identify postoperative complications and tumor recurrence. Physicians then conducted follow-up evaluations by telephone or in clinics.
Locoregional recurrence was defined as recurrence at the site of the anastomosis or the site of any of the surgeries. Cervical lymph node recurrence was treated as locoregional recurrence in patients with thoracic anastomosis. Distant recurrence was defined as tumor metastasis to distant organs or to any tissues that were not involved in surgery.
Statistical analysis
Descriptive statistics were used to describe patient characteristics and outcomes. The Shapiro-Wilk test was used to examine the normality of the data. All normally distributed data were analyzed for heterogeneity using one-way ANOVA with a multiple comparison post hoc test, and chi-squared tests were used for continuous and categorical variables. Non-normally distributed variables were analyzed using the Kruskal-Wallis test with adjustments for multiple comparisons. The Kaplan-Meier method was used to analyze long-term survival, and significant differences between the groups were identified by the log-rank test. A Cox-proportional multivariate analysis was used to identify the most significant mortality predictors. After a univariate analysis of each risk factor, those with P values <0.1 were selected for the multivariate model. All statistical analyses were performed using GraphPad Prism 7.0a (GraphPad software, La Jolla, CA, USA) and SPSS 24.0 (IBM, Armonk, NY, USA). A P value <0.05 was considered statistically significant.
Results
Patient baseline data
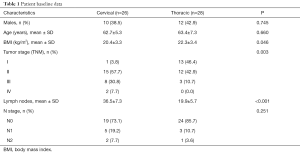
A total of 54 patients were enrolled in the study, including 26 with cervical anastomosis and 28 with thoracic anastomosis (Table 1). Significant differences were not observed in most baseline parameters, such as age and gender. The cervical anastomosis group had a lower mean body mass index (BMI) than the thoracic anastomosis group (20.4±3.3 vs. 22.3±3.4, P=0.046). Postoperative pathology suggested that the cervical anastomosis group included more stage III and IV patients (P=0.003) and that nearly half (46.4%) of the patients in the thoracic anastomosis group were classified as stage I. The patients in the cervical group also had a greater number of resected lymph nodes (36.5±7.3 vs. 19.9±5.7, P<0.001) and a higher positive lymph node rate than the thoracic group; however, the difference was not statistically significant (N1 + N2: 26.9% vs. 14.3%, P=0.251).
Full table
Postoperative results
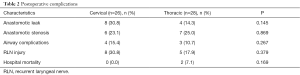
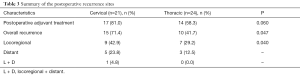
Two patients in the thoracic anastomosis group died during hospitalization. One died because of a severe infection caused by a postoperative anastomotic leak, and the other died due to pulmonary complications and the inability to extubate. No deaths occurred during hospitalization in the cervical anastomosis group. Additionally, there were no significant differences in postoperative complications, including anastomotic leak, anastomotic stenosis, pulmonary complications and RLN injury, between the two groups (Table 2). A greater number of patients underwent postoperative adjuvant radiotherapy and chemotherapy in the cervical anastomosis group, but the difference was not statistically significant (81.0% vs. 58.3%, P=0.060). Moreover, the cervical anastomosis group exhibited a higher recurrence rate (71.4% vs. 41.7%, P=0.047) and greater locoregional recurrence (P=0.040) than the thoracic anastomosis group (Table 3).
Full table
Full table
Long-term survival
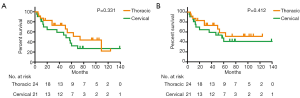
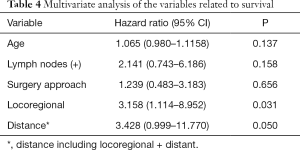
A total of 24 thoracic anastomosis and 21 cervical anastomosis patients were followed up. The follow-up period was between 3 and 148 months, and the mean follow-up time was 48.1 months. Although the thoracic anastomosis group had a longer median survival time (74 vs. 51 months), no significant difference in survival was observed between the two groups in either overall survival (P=0.331) or tumor-specific survival (P=0.412) (Figure 2). Cox regression analysis also confirmed that the surgical approach was not an independent risk factor for survival time (Table 4). However, postoperative recurrence (P=0.031, 95% CI: 1.114–8.952) was an independent risk factor for survival time, and distant recurrence (P=0.050, 95% CI: 0.999–11.770) may also affect survival time.
Full table
Discussion
The esophageal cancer segmentation criteria was revised in the most recent 8th edition of the AJCC/UICC TNM staging classification (4). The length of a tumor is now measured from the tumor epicenter to the incisors rather than to the upper edge of the tumor. The upper edge of the sternal notch is the anatomical landmark for cervical and upper thoracic tumors. Currently, the determination of tumor location and surgical approach relies on gastroscopy. Although the distance between a tumor and the incisors can be determined by gastroscopy, the relationship between tumor location and the sternal notch is not clear due to varying heights and chest wall configurations. Due to the shape of the thorax, the cervical esophagus segment in the chest is relatively long, which explains the anatomic basis of thoracic anastomosis for higher thoracic esophageal tumors. Therefore, for tumors located 18–22 cm from the incisors, thoracic anastomosis could be performed to reduce the risk of postoperative complications associated with cervical anastomosis. Minimally invasive surgery can also be considered (12). As squamous cell carcinoma is predominantly observed in Asian populations (1,6), we selected only these patients as the target screening group.
The national comprehensive cancer network (NCCN) requires at least 15 lymph nodes to be dissected during surgery, but it is not clear which regions should be scanned (13). Several studies have proposed that at least 23 lymph nodes should be dissected (14). Other reports (15,16) have also evaluated the number of dissected lymph nodes and found that the number of dissected lymph nodes is probably not associated with long-term survival. van der Schaaf et al. (17) reported that thoracic anastomosis patients with <7 dissected lymph nodes, 7–15 dissected lymph nodes and 16–114 dissected lymph nodes exhibited no significant differences in long-term survival. Lagergren et al. (3) distributed patients into four groups based on the number of dissected lymph nodes: 0–10, 11–14, 15–20 and 21–25. However, long-term follow-up revealed no significant differences among the groups regarding all-cause death or tumor-specific death. However, patients with more positive lymph nodes and a higher positive/negative rate were associated with a higher death rate. Both studies mentioned previous reports that showed that more extensive lymph node resection could improve prognosis, but this is likely due to the improved accuracy of positive lymph node detection, which can lead to a higher histological tumor stage. Our results agreed with this rationale, with cervical anastomosis patients exhibiting higher positive lymph nodes rates, although the difference was not statistically significant (26.9% vs. 14.3%, P=0.251). This lack of correlation between lymph node dissection and long-term survival is not unique to esophageal cancer. In breast, endometrial, pancreatic, gastric and rectal cancer, increased lymph node dissection did not correlate with greater benefit (3,18-20).
Based on these reports, studies have suggested that the dissection of high-value lymph nodes is much more important than the number of nodes dissected (21). Using the Japan Comprehensive Registry of Esophageal Cancer database (6), Tachimori et al. evaluated the efficacy of lymph node dissection by area. “Low efficacy index” lymph nodes showed limited predictive value for prognosis and staging, despite the dissection of more than 20 lymph nodes. In the efficacy index system, the mediastinal zone exhibited the greatest predictive value, especially bilateral RLN nodes. Other studies have proposed that RLN nodes can be predictors of cervical lymph node metastasis in squamous cell carcinoma (22-25). In our study, the low number of RLN+ patients prevented the analysis of differences between the two groups. However, no significant difference was observed in long-term survival when 2FL and 3FL RLN+ patients were compared (22). This may have occurred because the 3FL procedure cannot achieve the removal of all lymph nodes embedded along the tracheobronchial tree; therefore, it cannot reduce the lymph node recurrence rate, as micrometastasis occurs in 50% of N0 patients (26,27). To mitigate this limitation, greater radical lymph node dissection, such as complete mediastinal lymph node dissection via median sternotomy, may be required to reduce recurrence rates. However, current surgical technology cannot achieve this objective (27). Shim and colleagues (10) noted that many research studies have reported lower cervical lymph node metastasis rates than previous reports. Therefore, for patients in whom cervical metastasis was not detected in preoperative tests, 3FL dissection is not necessary.
Compared with 2FL dissection, another disadvantage of 3FL dissection is the increased rate of postoperative complications, including but not limited to pulmonary complications, anastomotic leak, anastomotic stenosis and RLN injury, which may affect quality of life (8,9). The highest reported incidence of RLN injury is 69% (10). Bilateral RLN resection may lead to RLN injury but can enable surgeons to more clearly resect RLNs. RLN injury not only slows recovery but also impedes speaking, swallowing and breathing and reduces long-term quality of life. Anastomotic leak and stenosis significantly extended the length of hospital stay and caused long-term pain in patients. In our study, cervical anastomosis patients exhibited a higher rate of complications than thoracic anastomosis patients, but this difference was not statistically significant, which was likely due to the small sample size. Additionally, cervical anastomosis patients suffered a higher rate of both locoregional and distant recurrence. This may due to the higher rate of stage III/V and positive lymph nodes.
Finally, our research has the following limitations. First, the limited patient number may have prevented the elucidation of differences between the two groups. Second, due to the retrospective nature of our study, we were not able to perform appropriate group baseline data matching, and thus, the higher number of advanced-stage patients in the cervical anastomosis group possibly reflects selection bias, which may affect the final conclusion. Third, neoadjuvant therapy is becoming increasingly more important for the comprehensive treatment of esophageal cancer. However, due to the extensive time span of included patients in our study, many different chemotherapy and radiotherapy strategies were employed; therefore, we excluded all patients who received chemotherapy or radiotherapy. Fourth, fewer lymph nodes were dissected in our study than in other studies, which could have affected the final outcomes. Lastly, we did not include thoracic shape in the statistical analysis, which may have affected the determination of tumor location in patients with esophageal cancer.
Conclusions
In this study, we retrospectively analyzed patients with cervicothoracic esophageal squamous cell carcinoma. Thoracic anastomosis with 2FL dissection achieved satisfactory outcomes compared with cervical anastomosis with 3FL dissection. No significant difference in long-term survival was observed between the two groups.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the institutional ethics board of Nanjing Drum Tower Hospital (2017-175-01) and written informed consent was obtained from all patients.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Lagergren J, Mattsson F, Zylstra J, et al. Extent of Lymphadenectomy and Prognosis After Esophageal Cancer Surgery. JAMA Surg 2016;151:32-9. [Crossref] [PubMed]
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-42.
- Walther B, Johansson J, Johnsson F, et al. Cervical or thoracic anastomosis after esophageal resection and gastric tube reconstruction: a prospective randomized trial comparing sutured neck anastomosis with stapled intrathoracic anastomosis. Ann Surg 2003;238:803-12; discussion 812-4. [Crossref] [PubMed]
- Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016;13:1-7. [Crossref] [PubMed]
- Isono K, Sato H, Nakayama K. Results of a nationwide study on the three-field lymph node dissection of esophageal cancer. Oncology 1991;48:411-20. [Crossref] [PubMed]
- Shang QX, Chen LQ, Hu WP, et al. Three-field lymph node dissection in treating the esophageal cancer. J Thorac Dis 2016;8:E1136-49. [Crossref] [PubMed]
- Ma GW, Situ DR, Ma QL, et al. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol 2014;20:18022-30. [Crossref] [PubMed]
- Shim YM, Kim HK, Kim K. Comparison of survival and recurrence pattern between two-field and three-field lymph node dissections for upper thoracic esophageal squamous cell carcinoma. J Thorac Oncol 2010;5:707-12. [Crossref] [PubMed]
- Nishihira T, Hirayama K, Mori S. A prospective randomized trial of extended cervical and superior mediastinal lymphadenectomy for carcinoma of the thoracic esophagus. Am J Surg 1998;175:47-51. [Crossref] [PubMed]
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Ajani JA, D'Amico TA, Almhanna K, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw 2015;13:194-227. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Chao YK, Liu HP, Hsieh MJ, et al. Impact of the number of lymph nodes sampled on outcome in ypT0N0 esophageal squamous cell carcinoma patients. J Surg Oncol 2012;106:436-40. [Crossref] [PubMed]
- Lagarde SM, Vrouenraets BC, Stassen LP, et al. Evidence-based surgical treatment of esophageal cancer: overview of high-quality studies. Ann Thorac Surg 2010;89:1319-26. [Crossref] [PubMed]
- van der Schaaf M, Johar A, Wijnhoven B, et al. Extent of lymph node removal during esophageal cancer surgery and survival. J Natl Cancer Inst 2015.107. [PubMed]
- Michalski CW, Kleeff J, Wente MN, et al. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br J Surg 2007;94:265-73. [Crossref] [PubMed]
- Georgiou P, Tan E, Gouvas N, et al. Extended lymphadenectomy versus conventional surgery for rectal cancer: a meta-analysis. Lancet Oncol 2009;10:1053-62. [Crossref] [PubMed]
- Jiang L, Yang KH, Chen Y, et al. Systematic review and meta-analysis of the effectiveness and safety of extended lymphadenectomy in patients with resectable gastric cancer. Br J Surg 2014;101:595-604. [Crossref] [PubMed]
- Miyata H, Yamasaki M, Makino T, et al. Therapeutic value of lymph node dissection for esophageal squamous cell carcinoma after neoadjuvant chemotherapy. J Surg Oncol 2015;112:60-5. [Crossref] [PubMed]
- Li H, Yang S, Zhang Y, et al. Thoracic recurrent laryngeal lymph node metastases predict cervical node metastases and benefit from three-field dissection in selected patients with thoracic esophageal squamous cell carcinoma. J Surg Oncol 2012;105:548-52. [Crossref] [PubMed]
- Kato H, Tachimori Y, Mizobuchi S, et al. Cervical, mediastinal, and abdominal lymph node dissection (three-field dissection) for superficial carcinoma of the thoracic esophagus. Cancer 1993;72:2879-82. [Crossref] [PubMed]
- Yoshioka S, Fujiwara Y, Sugita Y, et al. Real-time rapid reverse transcriptase-polymerase chain reaction for intraoperative diagnosis of lymph node micrometastasis: clinical application for cervical lymph node dissection in esophageal cancers. Surgery 2002;132:34-40. [Crossref] [PubMed]
- Ye K, Xu JH, Sun YF, et al. Characteristics and clinical significance of lymph node metastases near the recurrent laryngeal nerve from thoracic esophageal carcinoma. Genet Mol Res 2014;13:6411-9. [Crossref] [PubMed]
- Phillips AW, Lagarde SM, Navidi M, et al. Impact of Extent of Lymphadenectomy on Survival, Post Neoadjuvant Chemotherapy and Transthoracic Esophagectomy. Ann Surg 2017;265:750-6. [Crossref] [PubMed]
- Tachibana M, Kinugasa S, Yoshimura H, et al. Clinical outcomes of extended esophagectomy with three-field lymph node dissection for esophageal squamous cell carcinoma. Am J Surg 2005;189:98-109. [Crossref] [PubMed]