Boiling and roasting treatment affecting the peanut allergenicity
Introduction
Recently, 2% of the proportion of food allergies contributing to allergic diseases has increased (1,2). Peanuts are one of the most important food allergens in the world (3). Studies have shown that the incidence of peanut allergies in Western-born Asian is higher than Asians (4). Mostly Europeans and Americans eat roasted peanuts, whereas mostly Asians eat boiled and fried peanuts (5). Therefore, different processing methods may affect the sensitization of peanut products, but the specific mechanism underlying this change is not clear. The influence of thermal processing on peanut allergenicity is urgently required (6).
Food allergic responses are normally primed at intestinal mucosa, and the allergenicity reactions are triggered by the mast cells degranulation combined with specific immunoglobulin IgE (7). IgE-induced food allergy is a TH2-type allergic reaction. The major Th2-type cytokine IL-4 is critical to trigger IgE class switching (8). Hence, the relative concentrations of IgE, IgG, and cytokines are key to determine the strength of the food allergic reaction in mice model.
To date, 13 peanut allergens have been identified from Ara h 1 to Ara h 17 (9). Ara h 2 is the most important peanut allergen, which is the part of the vicilin and conglutin families of storage proteins (10). Ara h 2, similarly with the remaining allergens in the peanut, may interact with the intestinal epithelium differently when combined with their food matrix or in unison, thus allowing M cell-independent IEC transport across the intestinal epithelium (11). Thermal processing will be potential to modify the structure, solubility, and immunogenicity of peanut allergen Ara h 2 or other allergens (12). The conformation of food proteins is consisting of primary structure, secondary structure and tertiary structure. The formation of ɑ-helixes and β-sheets is promoted by interactions between polypeptide chains linked together by hydrophobic and hydrophilic interactions, electrostatic interactions and disulfide bonds (13). It means that all these chemical interactions will change the conformation and structure of the peanut proteins during heat treatment. Some research investigated the stability of soybean protein after the Maillard reaction by simulated gastrointestinal digestion (14). From a biochemical perspective, thermal processing promotes conformational changes of food proteins, and may further affect digestion and absorption of proteins or peptides by the intestinal epithelium, as well as their recognition by immune cells. Furthermore, if the sugars are present during the heat treatment, the free amino groups of side chains of amino acids can be blocked due to the Maillard reaction (15). Thus we speculate that the Maillard reaction alter immunoreactivity towards food allergen proteins. It is necessary to find the effect of different thermal processing on peanut allergenicity and the connection between the Maillard reaction and peanut allergic reaction.
In this study, we determine how different processes will affect peanut allergenicity with mice. Ethology, pathology and serology were observed to explore the differences in peanut allergenicity. Simulated gastrointestinal digestion experiments were conducted to determine the stability of proteins. Ultraviolet spectrum and CD spectra were used to detect structural changes in Ara h 2 allergens of boiled and roasted peanuts. The conclusions show that roasting and boiling process increase or decrease the allergenicity of peanuts.
Methods
Experimental materials
Some peanuts were purchased from Shanghai Wal-Mart Supermarket. Pathogen-free female BALB/c mice (4 weeks of age) were purchased from the animal experiment center at Fudan University.
Experimental reagents
Goat anti-mouse IgE, IgG2a and mouse IL-4, IFN-γ, histamine, and MCP-1 enzyme-linked immunosorbent assay (ELISA) kits were purchased from Fusheng Industrial Co., Ltd. (Shanghai, China). Skim milk powder, and TMB color liquid were purchased from Yi Sheng (Shanghai, China). And 4% paraformaldehyde fixed liquid was purchased from Qian Chen (Shanghai, China).
Experimental instruments
The experimental instruments used were as follows: single-function optical absorption microplate reader EMax Plus (Molecular Devices Corporation, USA); HCP-200-type high-speed multi-function crusher (Flag Arrow Shredders, Shanghai, China); ultrasonic grinder (Misonix Ltd Company, USA); Olympus BX51 microscope (Japan); constant temperature magnetic stirrer (Zhiwei Electrical Appliance Co, Ltd. Shanghai, China).
Experimental methods
Peanut preparation and thermal treatment
Peanuts were treated under one of the following three conditions: (I) boiling (100 °C, 20 min); (II) roasting (170 °C, 15 min). We added 0.2 g peanut product powder to phosphate buffer saline (PBS) buffer (20 mL) in a centrifuge tube. These were put in an ultrasonic grinding mill.
Animal sensitization experiments
BALB/c mice were randomly divided into four groups according to their body weight. The four groups were as follows: control (PBS), raw peanut, boiled peanut and roasted peanut. All mice were fed in an SPF level animal room for 10 days with free access to food and drinking water. The mice in the five groups were perfused with 0.5 mL (the amount of peanut was approximately 5 mg) sample as follows (Figure 1): a large gavage was performed on the 35th day where the amount of sample was increased to 25 mg. The weight and allergic symptoms of the mice was recorded every week.
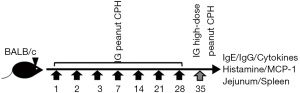
Determination of specific antibodies
On the 35th day, blood was collected from mice, stored in a tube containing anticoagulant, and left at 4 °C overnight. The serum was centrifuged at 3,000 ×g for 20 min and stored at −20 °C after packaging. The relative concentrations of specific antibodies (IgE, IgG1, IgG2a) were determined by ELISA.
Determination of cytokines
The cryopreserved serum was collected and the concentrations of IL-4 and IFN-γ were measured by ELISA.
Determination of histamine and MCP-1
The frozen serum was collected and the concentrations of histamine and MCP-1 were measured by ELISA.
Histopathological observations
Mice were sacrificed and blood was drawn after a large number of stimulations at 35 days. The mice were cervical dislocated. The spleen and approximately 1 cm of jejunum were removed. The jejunum of the mice was put into 4% paraformaldehyde fixative. H&E staining was performed to observe inflammatory infiltration of the jejunum.
Simulated gastric digestion of crude protein with different peanut products
First, 190 µL 1 M pepsin solution (Ph =1.2) was added to a 1.5 mL centrifuge tube at 37 °C for 5 min, and then 10 µL of 5 g/L sample protein and 70 µL of 0.2 mol/L sodium bicarbonate solution were vortexed and added to an ice bath. Then, 135 µL loading buffer was added and incubated in a boiling water bath for 5 min and cooled at room temperature. Next, 1.9 mL 1 M pepsin solution (pH =1.2) was added at 37 °C in a water bath for 5 min. Then, 100 µL of 5 g/L sample protein was vortexed and placed in a 37 °C water bath for 15 s, and 2, 30 and 60 min. At each time point, 200 µL of the reaction solution was added to a 1.5 mL centrifuge tube (containing 70 µL of 0.2 mol/L sodium bicarbonate solution) and placed into an ice bath. Then, 135 µL loading buffer was added and the sample was boiled in a water bath for 5 min then cooled at room temperature. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) samples were prepared at every time point.
Ultraviolet spectrum analysis
Ara h 2 proteins were purified from raw, roasted and boiled peanuts, which involved anion exchange spectrum using a DEAE-Sepharose Fast Flow column (16×250 nm), followed by 0.3–0.4 mol/L NaCl linear gradient elution, dialysis three times, concentration by 30 kD Millipore, and then storage at −20 °C. Ara h 2 protein was analyzed using a UV-vis spectrophotometer (UV759CRT) with a scanning wavelength of 250–420 nm and scanning rate of 100 nm/min.
CD spectroscopy
A Jasco J-815 spectropolarimeter (Jasco, MA, Japan) was used for CD measurements at room temperature. The concentration of was 10 µM for the far-UV region ranging from 190–250 nm. The ellipticity was recorded at a scanning speed of 500 nm/min, with accumulations of 3, and a bandwidth of 0.1 nm.
Statistical analysis
Data in the graphs are presented as mean value for the group ± standard error of the mean. Analyses of data were performed by Graph Pad Prism software (La Jolla, CA, USA). Before statistical analysis, all data from in vivo studies were transformed logarithmically and checked for normal distribution. Data were tested by one-way ANOVA with Bonferonni as post hoc test, if not otherwise mentioned. Differences were considered significant when P values were
Results
Effect of different peanut products on BALB/c mice ethology
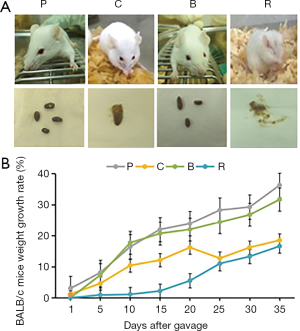
First of all, the appearance and excretion of the mice were observed. The mice from the roasted peanut groups exhibited scratching behavior and these mice also had diarrhea (Figure 2A). The weight of the mice was measured during the intragastric process. The weights growth rate of the different treatment groups was lower than that of the PBS group (Figure 2B). The weight of mice treated with roasted peanut was lowest in the other peanut treatment groups. The weight of mice treated with boiled peanuts were closed to that of the PBS group (Figure 2B). Feeding roasted and raw peanuts caused physical discomfort in mice such as diarrhea (Figure 2A), and resulted in retarded growth in the mice. These findings demonstrate that roasted peanut led to physical discomfort in mice, including diarrhea, and impaired weight gain. However, feeding boiled peanuts had little effect on mice ethology.
Effect of different peanut products on BALB/c mice pathology
Effect of different peanut products on jejunum structure and spleen
H&E stained paraffin sections of the fixed jejunal tissues were observed under a light microscope and photographed. Figure 3A shows that the villi in the treated jejunal structures were irregularly arranged, and the internal tissue was damaged greatly. This indicates that different peanut products cause allergic inflammation leading to jejunal damage.
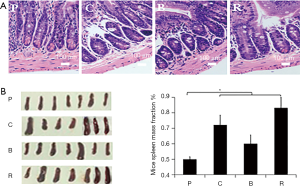
The spleen weight of mice treated with roasted peanut increased significantly (Figure 3B,C). The spleen weight of mice treated with roasted peanut was 0.3% greater than the PBS group. However, the spleen weight of mice treated with boiled peanuts were nearly 0.1% higher than the PBS group (Figure 3C). These results indicate that all peanut products increase the size of the spleen in mice, and implies that roasted peanut is more potential allergic than other peanut products. But boiled peanuts show a lower sensitization than raw peanuts.
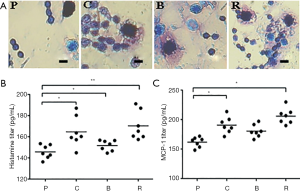
Effect of different peanut products on mice mast cells and basophil degranulation
The results showed that the degranulation of basophils in the PBS group was lesser than that in the other groups, and there was no halo around the cells (Figure 4A). The degranulation of mast cells in the PBS group was reduced compared to the other groups. In addition, mucosal mast cell degranulation was assessed by measuring MCP-1 in the serum. The degranulation of basophils and mast cells causes the release of histamines and we found that titers of MCP-1 and histamine in the sera of the roasted peanut group was the highest of all groups (Figure 4B,C), further indicating the enhanced allergenicity of peanuts after roasting. We also found that degranulated mast cells in the boiled peanuts was lower than raw and roasted peanut group (Figure 4B,C). It is found that roasted peanuts led to stronger allergic reaction and boiled peanuts will reduce the allergic reaction.
Effect of different peanut products on BALB/c mice serology
The relative concentrations of specific IgG1 and IgG2a in the treated mice were higher than those in the PBS (Figure 5A). The relative concentration of IgG1 was greater than that of IgG2a (IgG1/IgG2a >1). This indicates an inflammatory response to allergens.
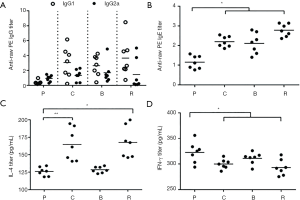
Generally, the amount of IgE in the serum indicates the allergic reaction. The results showed that the relative concentrations of IgE in boiled peanut groups were lower than the other peanut group; the relative concentration of IgE in the roasted peanut group was highest among the different peanut treated groups (Figure 5B). This indicates that different peanuts are allergenic and the allergenicity of boiled peanuts is weaker than raw and roasted peanuts.
Serum was taken from mice to determine IL-4 and IFN-γ levels by ELISA. The relative concentrations of IL-4 in the peanut treated groups were much higher than those of control. Particularly, the relative concentrations of IL-4 in the roasted and raw peanut groups were higher than those in the boiled peanut groups (Figure 5C), which indicated that roasted peanuts have a stronger allergenicity and boiled can reduce allergenicity. The TH1 type cytokine IFN-γ showed a trend opposite of IL-4 (Figure 5D). This result also indicated that this reaction is a TH2 type immune response, which causes inflammation. These results showed that raw and roasted peanuts are stronger allergenicity.
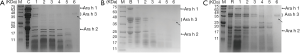
The stability of different peanut proteins in simulated gastrointestinal digestion
In order to study the mechanism of peanut sensitization, we also make further simulated gastrointestinal digestion experiments. The crude protein of raw, boiled and roasted peanuts were digested in simulated gastric fluid to observe the effect on peanut sensitizing proteins (Figure 6). These crude proteins underwent various degrees of degradation by simulated gastrointestinal digestion. Ara h 1 (65 kDa) and Ara h 3 (14–45 kDa) in raw and boiled peanut protein were significantly degraded by simulated gastric fluid for 15 s, and the Ara h 2 (17–20 kDa) protein was most degraded after digestion for 60 min (Figure 6A,B). The Ara h 1 and Ara h 3 proteins in roasted peanuts were significantly more resistant to digestion compared with raw and boiled peanuts, and the Ara h 3 protein was not digested after 2 min digestion. Ara h 2 bands were observed after digestion for 60 min (Figure 6C) and a new enzyme resistant band was present at 25 kDa after 30 min digestion. It was concluded that the sensitized protein in the roasted peanut was more resistant to pepsin tolerance than that of the raw peanut, indicating roasted peanuts have a greater potential sensitization.
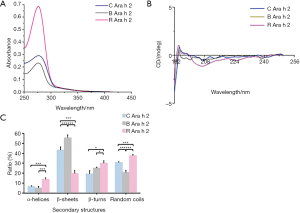
Effects of thermal processing on the Ara h 2 allergen structure of peanuts
The UV spectrum was applied to analyze the tertiary structures of Ara h 2 in raw, boiled, roasted peanuts (Figure 7A). Absorbance values of Ara h 2 at 280 nm were increased after roasting and reduced after boiling, indicating that thermal processing causes extensive denaturation and destruction of the tertiary structure, and might expose amino acid residues embedded in Ara h 2. Far-UV CD spectra was used to analyze the secondary structures of Ara h 2 (Figure 7B). The percentage of conformational units was calculated using CDPro software (Figure 7C). Compare to raw peanuts, the content of conformational units changed after roasting and boiling. A significant shift in roasted and boiled Ara h 2 was observed. The content of the β-sheet was decreased from 43.2% to 19.8%, and the α-helices, turn, and random coil content increased from 6.6% to 13.9%, 19.3% to 30.1%, and 30.7% to 37.6%, respectively in roasted Ara h 2. The content of the β-sheet and β-turn were increased from 43.2% to 55.6% and 19.3% to 25.1%, the α-helices and random coils content decreased from 6.6% to 5.04%, and 30.7% to 20.6%, respectively in boiled Ara h 2. These results show that the secondary structures were changed after roasting and boiling.
Discussion
In this study, we observed the weight of mice and their physical condition. When the mice were fed different peanuts, the weight growth rate was lower than the control (Figure 2). This may be due to allergy, at the same time, and we also found that raw peanut and roasted peanuts cause diarrhea. Compared with other peanut treated groups, the symptoms of weight loss and diarrhea caused by roasted peanut were more obvious. It was speculated that roasted peanut had stronger allergenicity than other peanut products.
Besides the discussion on mice behavior, the allergenicity of different peanuts as it related to pathogenicity in BALB/c mice was also studied. In this study, jejunal structure was observed and a various degree of damage after feeding different peanuts was found (Figure 3A). When the jejunum is not inflamed, the jejunal epithelial cells and mesentery are complete and the intestinal villi are clearly visible. Jejunal structure and intestinal villi will be destroyed following inflammation, which can even lead to the disappearance of intestinal villi (16). The spleen contains a large number of lymphocytes and macrophages in the body. Hence, allergic reactions will increase spleen enlargement, which was also verified by our experiment (Figure 3B,C). In this study, mouse jejunal structure showed various degrees of damage, mast cell and basophil degranulation, and various degrees of swelling of the spleen after feeding different peanuts. In this research, mast cell and basophil degranulation were observed in treated groups (Figure 4A). The concentration of histamine increased in different treatment groups, the concentration of histamine increased most obviously after feeding roasted peanut and the concentration of histamine in boiled group was lower than that in raw and roasted group (Figure 4B). Mast cell degranulation produces a large amount of histamine, which causes allergic reactions to become more intense. Thus, increased histamine indicates an enhanced allergic reaction. Mucosal mast cell degranulation was assessed by measuring MCP-1 in the serum. The relative concentration of MCP-1 in boiled peanut groups were lower than the other peanut treated groups, and then the relative concentration of MCP-1 in roasted peanut group was highest (Figure 4C). This indicates that the allergenicity of roasted peanut was the stronger than boiled peanut.
In this research, the serum concentration of IgE following roasted peanut treatment was greater than other peanut treatments, and the fact that IgG1/IgG2a >1 shows that the reaction is biased towards the TH2 allergic reaction (Figure 5A). Under normal conditions, Th1/Th2 cells are relatively balanced in mice (17). When this balance tips towards TH2 type cells, which is primarily caused by an allergic reaction in mice, a series of further allergic reactions, such as splenomegaly, mast cell degranulation, increased IgE levels, etc. is common (17). IgE is an immunoglobulin whose immune function is combined with mast cells and basophils (18). Therefore, the serum concentration of IgE is the key to determining allergenicity (19). Here, the relative concentration of IL-4 in the roasted peanut group was greater than other peanut products, while the relative concentration of IFN-γ in the roasted peanut group was lower compared to the other peanut products (Figure 5C). In the process of allergic reactions, some cytokines promote the process of sensitization or inhibition and include IL-4, a TH2-type cytokine, which plays an important role in promoting allergic reactions, which increase in parallel to the IL-4 concentration. IFN-γ is a TH1-type cytokine. IFN-γ has an inhibitory effect on the allergic response. Thus, a decrease in IFN-γ implies an increased allergic response (20). Reduced IFN-γ indicates that the hypersensitivity is enhanced, so we speculate that the roasted peanuts have the stronger sensitization and boiled peanuts can reduce the sensitization. These results indicated that roasted peanut is more allergenic than other peanuts and causes a stronger allergic reaction. Boiled peanut causes a weaker allergic reaction.
Protein digestion is commonly evaluated to assess allergenicity. Roasted peanut protein had a greater stability compared to raw and boiled peanut proteins in simulated gastric fluid (SGF) (Figure 6). Therefore, the high stability of roasted peanut protein might explain its high allergenicity. Furthermore, as protein structure is thought to be the basis of protein function, changes in allergen conformation might alter allergenicity (21). At high temperatures (above 90 °C), the Maillard reaction leads to the formation of advanced glycation end products (AGEs) in the food matrix, causing chemical modifications (5). These processes were further validated by highly stable roasted peanuts protein in SGF and Ultraviolet spectrum and CD spectra analyses. The UV spectrum was applied to analyze the structure of Ara h 2 from raw, boiled and roasted peanuts. The absorbance of Ara h 2 from raw peanuts at 280 nm were increased after roasting and were decreased after boiling (Figure 7A). It indicates that roasting and boiling resulted in the extensive denaturation and destruction of the tertiary structure, and possible exposure or degradation of parts of the amino acid residues. CD spectra was used to analyze the secondary structure of peanut allergens (Figure 7B,C). The results showed that α-helices, β-sheets, turns, and random coils were 6.6%, 43.2%, 19.3% and 30.7%, respectively in raw peanuts. After roasting, the ratios of the secondary structures were changed to 5.04%, 55.5%, 25.1% and 20.6%, respectively in roasted peanuts. After boiling, the ratios of the secondary structures were changed to 13.9%, 19.8%, 30.1% and 37.6%, respectively in boiled peanuts. It indicates the secondary structure of raw peanuts was more susceptible to change after roasting and boiling (Figure 7B,C). In addition to turns, other secondary structure trends are the opposite after roasting and boiling. The results of mice experiments suggest that different thermal processing affect the peanut allergenicity and roasting or boiling causes a marked alteration in structures of the major peanut Ara h 2 allergens compared to raw peanuts, which increased or reduced allergenicity. From a protein digestibility perspective, these results indicate that heating and Maillard reaction alter the proteins in gastrointestinal digestion due to unfolding, heat-induced disulfide bond interchanges, aggregation and formation of AGEs. Hence, we believe that the Maillard reaction may play a crucial role in the enhancing of immunogenic food allergens.
Conclusions
In this paper, changes in the indexes of mice were studied in response to different peanuts (Figure 8). The results showed that different processed peanut products could induce different levels of allergenicity in mice. The concentration of TH2-type serological indexes in roasted peanut was the stronger than those in raw peanut, but these indexes in boiled peanuts were weaker than those in raw peanut. The results support that the changes of Ara h 2 structure are related to the immunogenicity and roasting enhance the allergic reaction, while boiling reduce the allergic reaction in thermal process. We anticipate that our research could aid in improving the processing of peanut products and help reduce the harm of peanuts to sensitized people.
Acknowledgments
Funding: This study is supported by the National Key Research and Development Program of China (No.2016YFD0501101), the project of Food Science Discipline Construction of Shanghai University and the National Natural Science Foundation of China (No. 31201306).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This protocol was reviewed and approved by the appropriate Institutional Review Board (IRB) of Shanghai University (ID: 2018010) for experimental animals. All surgery was performed under sodium pentobarbital anesthesia, and all efforts were made to minimize suffering.
References
- Koplin JJ, Allen KJ, Gurrin LC, et al. The Impact of Family History of Allergy on Risk of Food Allergy: A Population-Based Study of Infants. Int J Environ Res Public Health 2013;10:5364-77. [Crossref] [PubMed]
- Marco-Martín G, La RHA. Differences in the Anaphylactic Response between C3H/HeOuJ and BALB/c Mice. Int Arch Allergy Immunol 2017;173:204. [Crossref] [PubMed]
- Custovic A. To what extent is allergen exposure a risk factor for the development of allergic disease? Clin Exp Allergy 2015;45:54-62. [Crossref] [PubMed]
- Shek LP, Cabrera-Morales EA, Soh SE, et al. A population-based questionnaire survey on the prevalence of peanut, tree nut, and shellfish allergy in 2 Asian populations. J Allergy Clin Immun 2010;126:324-31, 331.e1-7.
- Hebling CM, Mcfarland MA, Callahan JH, et al. Global proteomic screening of protein allergens and advanced glycation endproducts in thermally processed peanuts. J Agric Food Chem 2013;61:5638-48. [Crossref] [PubMed]
- Moghaddam AE, Hillson WR, Noti M, et al. Dry roasting enhances peanut allergic sensitization across mucosal and cutaneous routes in mice. J Allergy Clin Immun 2014;134:1453. [Crossref] [PubMed]
- Zhou C, Ludmila T, Sun N, et al. BALB/c mice can be used to evaluate allergenicity of different food protein extracts. Food Agr Immunol 2016;27:1-15. [Crossref]
- Huang CH, Lin YC, Jan TR. Lactobacillus reuteri induces intestinal immune tolerance against food allergy in mice. J Funct Foods 2017;31:44-51. [Crossref]
- Rao H, Tian Y, Tao S, et al. Key factors affecting the immunoreactivity of roasted and boiled peanuts: Temperature and water. Lwt-Food Sci Technol 2016;72:492-500. [Crossref]
- Koppelman SJ, Wensing M, Ertmann M, et al. Relevance of Ara h1, Ara h2 and Ara h3 in peanut-allergic patients, as determined by immunoglobulin E Western blotting, basophil-histamine release and intracutaneous testing: Ara h2 is the most important peanut allergen. Clin Exp Allergy 2004;34:583-90. [Crossref] [PubMed]
- Price DB, Ackland ML, Burks W, et al. Suphioglu C. Peanut allergens alter intestinal barrier permeability and tight junction localisation in Caco-2 cell cultures. Cell Physiol Biochem 2014;33:1758-77. [Crossref] [PubMed]
- Shen LL, Zhu QQ, Huang FW, et al. Effect of heat treatment on structure and immunogenicity of recombinant peanut protein Ara h 2.01. Lwt-Food Sci Technol 2015;60:964-9. [Crossref]
- Somoza V, Wenzel E, Weiss C, et al. Dose-dependent utilisation of casein-linked lysinoalanine, N(epsilon)-fructoselysine and N(epsilon)-carboxymethyllysine in rats. Mol Nutr Food Res 2006;50:833-41. [Crossref] [PubMed]
- Yang Y, Cui SW, Gong J, et al. A soy protein-polysaccharides Maillard reaction product enhanced the physical stability of oil-in-water emulsions containing citral. Food Hydrocoll 2015;48:155-64. [Crossref]
- Hellwig M, Henle T. ChemInform Abstract: Baking, Ageing, Diabetes: A Short History of the Maillard Reaction. Angew Chem Int Ed Engl. 2014;53:10316-29. [Crossref] [PubMed]
- Noti M, Kim BS, Siracusa MC, et al. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J Allergy Clin Immun 2014;133:1390-9. [Crossref] [PubMed]
- Shin HS, See HJ, Jung SY, et al. Turmeric (Curcuma longa) attenuates food allergy symptoms by regulating type 1/type 2 helper T cells (Th1/Th2) balance in a mouse model of food allergy. J Ethnopharmacol 2015;175:21-9. [Crossref] [PubMed]
- Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol 2010;10:440-52. [Crossref] [PubMed]
- Pali-Schöll I, Jensen-Jarolim E. Basic Aspects of Allergy and Hypersensitivity Reactions. Tokyo: Springer, 2009:3-17.
- Brooks BM, Thomas AL, Coleman JW. Benzylpenicillin differentially conjugates to IFN- γ, TNF- α, IL-1 β, IL-4 and IL-13 but selectively reduces IFN- γ activity. Clin Exp Immunol 2003;131:268-74. [Crossref] [PubMed]
- Achouri A, Boye JI. Thermal processing, salt and high pressure treatment effects on molecular structure and antigenicity of sesame protein isolate. Food Res Int 2013;53:240-51. [Crossref]