Ten quality indicators for endoscopic submucosal dissection: what should be monitored and reported to improve quality
Introduction
Endoscopic submucosal dissection (ESD) is an endoscopic interventional procedure initially proposed in Japan about 20 years ago and subsequently became popular in Asia and, more recently, also in Europe and North America. This technique aims to achieve en bloc resection of superficial neoplasia located in the upper and lower gastrointestinal (GI) tract (1-3). The key performance indicators (KPI) and the monitoring of quality of procedure has never been addressed formally. However due to its increasing popularity even outside tertiary, high-volume, referral centers, there is an urgent need to identify quality indicators to implement as part of internal audit process. In addition, the number of publications have progressively increased. Several thousand publications have been indexed in the main electronic databases, (e.g., Medline, EMBASE and Scopus). We have systematically reviewed most of the published studies and found that the reporting quality was poor in most of the studies (4,5). Several important information and data points were missing or incompletely reported, thus limiting the interpretation of the results.
Aim of the current review is to suggest a list of quality indicators that should constantly be monitored and accurately reported in the publication, in order to improve the reporting quality and the usefulness of the publication. Every endoscopist performing ESD should monitor these parameters and should be able to provide such information as part of a quality control. For the purpose of the current review, we will refer to the lower GI tract, but our suggestions and conclusions equally apply to the upper GI tract.
Quality indicators that should be monitored and reported in every ESD publication
In the following paragraphs, we will briefly discuss which items should be included in every publication based on the ESD procedure and the reasons why these items are important for the correct understanding of the publication and why they should be considered as quality indicators (Table 1).

Full table
Indications for ESD
ESD is mainly performed for three indications: (I) large, >20 mm in diameter, lesions for which endoscopic treatment is indicated but for which en bloc resection by snare would be difficult; (II) mucosal lesions with fibrosis (e.g., because of prior biopsy) and, (III) recurrence after prior endoscopic resection. Outcomes may vary broadly, because the dissection of lesions with fibrosis, especially in the setting of recurrence, is very challenging, requiring longer procedure time and prone to complications than ESD performed in naïve lesions without fibrosis (6,7). In the study by Hori et al. (6), 247 lesions dissected by ESD were analysed with the aim to identify risk factors for prediction of technical difficulty and found that tumour with scarring or locally recurrent was a risk factor for longer procedure duration (OR 4.7; 95% CI: 1.7 – 13.7), and for switching from en-bloc to piecemeal resection (OR 7.8; 95% CI: 2.4 – 25.0). Main outcomes, such as en bloc and R0 resection rates as well as perforation rates, might be worse than expected, as outlined in Table 2. In this table, we reported main outcomes after ESD extracted from studies including only patients with recurrent lesions post endoscopic resection attempt (8-14). Worst outcomes might be more evident at the beginning of the learning curve phase or in low-volume centres.
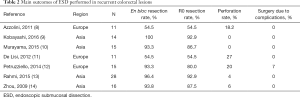
Full table
Most of the published studies did not stratify the results according to the indication, thus including heterogeneity in the study results and difficult interpretation.
Use of morphological criteria for the evaluation of the lesions (e.g., Paris) and classifications [Japan NBI Expert Team (JNET), NBI International Colorectal Endoscopic (NICE) classification] used for optical diagnosis should be clearly documented before ESD
ESD should be restricted to lesions highly suspicious for superficial malignant invasion (1) but in more than 80% of the cases it is used for the dissection of adenomas with low- (20–40%) or high-grade dysplasia (40–60%) (5). In almost all series, advanced endoscopic imaging techniques and current classifications proposed with the intent to stratify the risk of malignancy and depth of invasion are implemented for interrogating and correctly selecting the lesion to dissect. However, documentation of these classifications and pre-ESD diagnosis is not very clear and standardised. Inclusion of morphological criteria will allow us to get some real-time data of the use of these classifications outside the research settings. In fact, data from validation exercises (15-18) look very good but their external validity outside controlled setting should be verified.
The supposed diagnosis before ESD and the classification adopted should be reported and a periodic assessment of the concordance with histology should be carried out.
En bloc, R0 and oncologically curative resection rates
The main difference between ESD and piece-meal endoscopic resection (EMR) is the possibility to achieve en bloc resection, regardless of the size of the lesion. Therefore, it is almost obvious that the en bloc resection rate is the main quality indicator of the technical performance. During the learning curve, en bloc resection rate of 80% can be achieved after 5 ESDs in the rectum and 20 ESDs in the colon (19), however there could be great variability (20,21). Overall, an en bloc resection rate above 90% has been reported, however there is still a significant difference between Western (81.2%; 95% CI: 77.1–84.7%) and Eastern regions (93.0%; 95% CI: 91.4–94.3%) (4). This indicator is easy to assess and helpful to monitor personal progress and achievement during time.
More important than the en bloc resection rate is the R0 resection rate, since it represents the ability to perform oncologically complete dissection. R0 is defined as both vertical and horizontal margins free of neoplasia; surely the evaluation of the vertical margin is the most important, since it allows the adequate assessment of the deep of invasion. It also indirectly represents a performance indicator of the ability to dissect the submucosal layers and correctly identify and circumscribe the lesion. In addition, R1 resection with vertical margin positive for neoplastic tissue, might represent an incorrect case selection, because of massive invasion of deeper submucosal (SM) layers. A R0 resection rate above 80% is generally stated and should be considered a reliable cut-off value but, again, a significant difference between Western (71.3%; 95% CI: 66.2–75.9%) and Eastern 85.6% (95% CI: 83.3–87.7%) countries has been reported (4).
The oncologically curative resection is defined when several features are present: no lymphatic and vascular invasion, well-differentiated (G0/G1) tumor and proper distance from vertical margin, and malignant invasion limited to sm1 or <1,000 mm. This parameter is very important because it is the only parameter that can completely show the usefulness of ESD in the management of early cancer as alternative to surgery. Based on the available data, the oncologically curative resection rate is about 75%, with a number needed to treat (NNT) with ESD to avoid one surgery for malignant lesions of about 17 (5). Unfortunately, this outcome is rarely reported.
Histology (adenoma low- and high-grade dysplasia vs. cancer, sm1 vs. sm2 or deeper)
As previously mentioned, most of the dissected lesions represent low- and high-grade adenomas; in addition, only half of the malignant lesions dissected is confined to the sm1 layer (5). Every endoscopist performing ESD should know the histology of own series, because it represents an indicator of the ability of case selection. Of particular importance is the sm1/sm2 or deeper rate which represents the ability to interrogate the lesion and discriminate superficial vs. massively invading cancer. Based on the best available evidence, the reported sm1/sm2 or deeper rate is about 50%; efforts should be carried out to increase this rate as close as possible to 100%.
Technique used: standard vs. hybrid ESD technique
The standard technique of ESD is based on the use of an ESD knife to perform the mucosal incision followed by submucosal dissection to achieve an en bloc resection. Since this technique is challenging, time-consuming and prone to complications, a less-challenging, faster and possibly safer alternative technique has been suggested, called hybrid, or knife-assisted snare resection (KAR) which is based on the sequential use of a knife and a snare (22). These techniques cannot be considered equivalent, indeed main outcomes like en bloc and R0 resection rates are significantly different between these techniques. The analysis of studies comparing the two techniques showed that the standard ESD technique achieves higher R0 resection rates than that observed with the hybrid technique (OR 2.44; 95% CI: 1.23–4.85) (4). The differences may be due to a variety of reasons like differences in case selection, experience and skills of the endoscopist but it is also likely that inherent differences in the two techniques could contribute to the differences in outcome. Therefore, it is important to highlight in the studies which technique was used to dissect and stratify the main outcomes accordingly. It would also be useful to report how many procedures were started with the intention of standard ESD approach but later got converted to the hybrid one and the reasons behind the change in technique.
Complications (need for surgery, perforation, bleeding, length of hospital stay)
The complication rate represents one of the main outcome measure and should always be monitored and reported in any article on ESD. In particular, a distinction between intra-procedural and late-onset complications should always be highlighted. This index usually changes during the learning curve, progressively reducing until reaching a plateau value; however, a late increase might be observed as the complexity and difficulty of the selected cases increase. Most of the complications can be conservatively managed during the procedure, such as small perforation and bleeding, therefore it is also important to monitor and report how many complications require a surgical approach. These latter may be considered as severe complications. Another important outcome measure that should be reported is the length of hospital stay after the development of a complication, because it represents an indirect index of the severity of complications and it is also very useful when performing cost-efficacy analysis. There is a significant difference in the rates of adverse events requiring surgery between Eastern (1.1%; 95% CI: 0.9–1.3%) and Western regions (3.1%; 95% CI: 2.1–4.7%), this seems partly due to the different volume of ESD carried out per year per center; indeed, low-volume centers (e.g., performing less than 25 ESDs/year) have higher rate of adverse events than high volume centers (4).
Volume of ESDs performed per year
As previously mentioned, the volume of ESDs performed per year is very important as most of the outcome measures strictly and directly correlate with the volume of ESDs carried out (4). It is important to note that total volume is important but it is also important to note volume per location of lesions.
Lesion location
It is important to stratify the volume of ESDs according to the site as the level of expertise required, depends on the location of lesions. It is well known that lesions located in the gastric antrum and the rectum can be approached more easily than lesions placed in the esophagus, gastric fundus or colon (3).
It is surprising that most of the studies reporting outcomes of the ESD procedure, especially in the lower GI tract, did not stratify the results according to the lesion location (e.g., rectum vs. colon), despite as previously stated, different level of difficulties and outcomes may be expected (4).
Need for surgery after technically successful ESD
Despite a successful ESD procedure, the histology may recommend the need for surgery because an oncologically curative resection is not guaranteed. This is an important outcome measure as it gives an insight into the lesion selection skills of the endoscopist and also into the real potential of ESD as a true alternative to surgery. Based on the available data, when the analyses are focused only on technically successful ESD, the NNT by ESD to spare one surgical resection is about 12–13, but it increases up to 16–17 when calculation is restricted to oncologically curative resection (5).
Time taken to perform the procedure
The total procedure should be monitored and reported because it indirectly correlates with the difficulty of the procedure and also with the ability and experience of the operator; this is particularly true during the learning phase (19,23). It will also give us an insight in future planning and scheduling of procedures and cost-effectiveness of ESD as compared to surgery.
Conclusions
In the last decade, ESD has become more popular, however the issue of quality control has never been raised. Therefore, there is an urgent need to identify possible quality indicators to monitor as part of internal audit process. This is particularly compelling, since the diffusion of ESD outside Asian, super-expert, high-volume, tertiary referral centers. Indeed, it has been clearly demonstrated that outcomes in Western regions are worse than those observed in Eastern regions. In addition, most of the published studies present a poor reporting quality, thus in most of the cases it is not clear how outcomes are influenced by confounding factors.
In the current review, we raised the issue of quality control for ESD and proposed a list of possible quality indicators that should be monitored by each endoscopist and reported in every study reporting results on ESD procedures. We feel that these quality indicators should be used in clinical practice by endoscopists to benchmark the data with the internationally recommended standards.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, et al. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2015;47:829-54. [Crossref] [PubMed]
- Longcroft-Wheaton G, Bhandari P. Management of early colonic neoplasia: where are we now and where are we heading? Expert Rev Gastroenterol Hepatol 2017;11:227-36. [PubMed]
- Fuccio L, Ponchon T. Colorectal endoscopic submucosal dissection (ESD). Best Pract Res Clin Gastroenterol 2017;31:473-80. [Crossref] [PubMed]
- Fuccio L, Hassan C, Ponchon T, et al. Clinical outcomes after endoscopic submucosal dissection for colorectal neoplasia: a systematic review and meta-analysis. Gastrointest Endosc 2017;86:74-86.e17. [Crossref] [PubMed]
- Fuccio L, Repici A, Hassan C, et al. Why attempt en bloc resection of non-pedunculated colorectal adenomas? A systematic review of the prevalence of superficial submucosal invasive cancer after endoscopic submucosal dissection. Gut 2018;67:1464-74. [PubMed]
- Hori K, Uraoka T, Harada K, et al. Predictive factors for technically difficult endoscopic submucosal dissection in the colorectum. Endoscopy 2014;46:862-70. [Crossref] [PubMed]
- Youk EG, Sohn DK, Hong CW, et al. Early Outcomes of Endoscopic Submucosal Dissection for Colorectal Neoplasms According to Clinical Indications. Dis Colon Rectum 2016;59:403-10. [Crossref] [PubMed]
- Azzolini F, Camellini L, Sassatelli R, et al. Endoscopic submucosal dissection of scar-embedded rectal polyps: A prospective study (Esd in scar-embedded rectal polyps). Clin Res Hepatol Gastroenterol 2011;35:572-9. [Crossref] [PubMed]
- Kobayashi N, Konishi J, Konno M. Su1680 Efficacy and Safety of Endoscopic Submucosal Dissection for Residual or Recurrence of Colorectal Neoplasms After Endoscopic Treatment. Gastrointest Endosc 2016;83:AB392. [Crossref]
- Murayama K, Sunada K, Hayashi Y, et al. The “pocket-creation method” facilitates ESD of recurrent colorectal lesions. J Gastroenterol Hepatol 2015;30:178.
- De Lisi S, Fiori G, Ravizza D, et al. P.13.7 Endoscopic submucosal dissection (ESD) for residual or recurrent colorectal adenomas: a single center experience. Dig Liver Dis 2012;44:S170. [Crossref]
- Petruzziello L, Greco S, Vitale G, et al. OC.21.2 Colorectal endoscopic submucosal dissection (CR-ESD) of residual/recurrent superficial neoplastic lesions after endoscopic or surgical resection. Retrospective analysis and outcomes. Dig Liver Dis 2014;46:S45. [Crossref]
- Rahmi G, Tanaka S, Ohara Y, et al. Efficacy of endoscopic submucosal dissection for residual or recurrent superficial colorectal tumors after endoscopic mucosal resection. J Dig Dis 2015;16:14-21. [Crossref] [PubMed]
- Zhou PH, Yao LQ, Qin XY. Endoscopic submucosal dissection for colorectal epithelial neoplasm. Surg Endosc 2009;23:1546-51. [Crossref] [PubMed]
- Komeda Y, Kashida H, Sakurai T, et al. Magnifying Narrow Band Imaging (NBI) for the Diagnosis of Localized Colorectal Lesions Using the Japan NBI Expert Team (JNET) Classification. Oncology 2017;93:49-54. [Crossref] [PubMed]
- Sumimoto K, Tanaka S, Shigita K, et al. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc 2017;85:816-21. [Crossref] [PubMed]
- Sumimoto K, Tanaka S, Shigita K, et al. Diagnostic performance of Japan NBI Expert Team classification for differentiation among noninvasive, superficially invasive, and deeply invasive colorectal neoplasia. Gastrointest Endosc 2017;86:700-9. [Crossref] [PubMed]
- Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc 2016;28:526-33. [Crossref] [PubMed]
- Iacopini F, Bella A, Costamagna G, et al. Stepwise training in rectal and colonic endoscopic submucosal dissection with differentiated learning curves. Gastrointest Endosc 2012;76:1188-96. [Crossref] [PubMed]
- Sakamoto T, Saito Y, Fukunaga S, et al. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum 2011;54:1307-12. [Crossref] [PubMed]
- Jeon HH, Lee HS, Youn YH, et al. Learning curve analysis of colorectal endoscopic submucosal dissection (ESD) for laterally spreading tumors by endoscopists experienced in gastric ESD. Surg Endosc 2016;30:2422-30. [Crossref] [PubMed]
- Chedgy FJ, Bhattacharyya R, Kandiah K, et al. Knife-assisted snare resection: a novel technique for resection of scarred polyps in the colon. Endoscopy 2016;48:277-80. [Crossref] [PubMed]
- Shiga H, Ohba R, Matsuhashi T, et al. Feasibility of colorectal endoscopic submucosal dissection (ESD) carried out by endoscopists with no or little experience in gastric ESD. Dig Endosc 2017;29 Suppl 2:58-65. [Crossref] [PubMed]


