Mechanical ventilation management during cardiothoracic surgery: an open challenge
Introduction
Mechanical ventilation is a technique used to support patient breathing. In the intensive care unit, we often need to restore a patient to a normal situation from a critical one. During surgery, the anesthesiologist must take care to do the patient no harm, particularly when using the ventilator. This is difficult during abdominal surgery, and even more so when patients have to undergo cardiothoracic anesthesia, often with an open chest and, at times, a collapsed lung. Indeed, nowadays up to 25% of patients develop postoperative pulmonary complications (PPC) following cardiothoracic surgery (1).
Peculiarities of mechanical ventilation in cardiothoracic surgery
Sternotomy and sternosynthesis
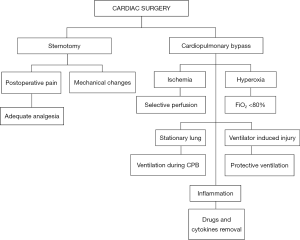
Sternotomy is one of the main alterations that takes place during cardiac surgery, and can be performed with either an open or closed pleura. In the latter, respiratory system elastance (Ers) remains unchanged, unlike following pleurotomy, due to an increase in chest wall elastance (Ecw) and a decrease in lung elastance (El) that occur simultaneously and balance each other out (Ers = Ecw + El) (2). The postoperative effects of sternotomy and subsequent sternosynthesis are burdened with complications: mechanical impairment to thoracic expansion (3) and the presence of postoperative pain, which can affect breathing by decreasing the protective coughing reflex, resulting in an increase of PPCs (4).
Nerve injury and ventilatory dysfunction
Paralysis of the phrenic nerve, whose incidence can vary from 1% to 60%, may affect postoperative recovery and increase PPC incidence by directly affecting diaphragm function (5).
A lesion of the intercostal nerves can lead to postoperative neuropathy, which can interfere with coughing and deep breathing. However, to date no studies have shown any correlation between intercostal neuralgia and PPC increase (6).
Cardiopulmonary bypass (CPB)
CPB is a pivotal technique in cardiac surgery. It allows surgeons to operate in a bloodless field, while blood flow and oxygenation are maintained. Nonetheless, it can cause various non-physiological alterations. Hemodilution, although contained when using a miniaturized circuit, is still required to avoid embolism and to reduce the hematocrit in order to avoid hemolysis. This, however, can cause pulmonary edema when the hematocrit reaches 22% (7). An extremely low hematocrit can provoke the need for blood transfusion, and consequently increase the possibility of adverse reactions such as transfusion-related acute lung injury (TRALI) (8).
The flow of blood through the CPB circuit releases inflammatory cytokines that cause lung damage, both directly and indirectly, by increasing the permeability of the interstitium (1). This, combined with the volume overload, increases the incidence of interstitial edema and consequent atelectasis (9). Furthermore, standard CPB circuits do not permit selective lung perfusion. As a result they receive up to 10 times less flow than usual (10), with a subsequent ischemic and reperfusion injury that will add to the inflammatory process caused by the CPB. This damage is further increased by the use and weaning from high FiO2 during CPB (11). An ulterior culprit might be lung standstill during CPB, which most likely causes the activation of lysosomal enzymes and impedes lymphatic drainage (12,13).
A study by Canet et al. (14) helps us define the pulmonary complications that can ensue from perioperative medicine:
- Respiratory infection: the patient has received antibiotics for a suspected respiratory infection, meeting one or more of the following criteria: new or changed sputum, new or changed lung opacities, fever, white blood cell count >12,000/mm3;
- Respiratory failure: postoperative PaO2 <60 mmHg on room air, a PaO2/FiO2 ratio <300 mmHg or arterial oxyhemoglobin saturation measured with pulse oximetry <90% and requiring oxygen therapy;
- Pleural effusion: chest X-ray showing blunting of the costophrenic angle, loss of sharp silhouette of the ipsilateral hemidiaphragm in an upright position, evidence of displacement of adjacent anatomical structures, or (in a supine position) a hazy opacity in one hemithorax with preserved vascular shadows;
- Atelectasis: lung opacification with a shift of the mediastinum, hilum, or hemidiaphragm toward the affected area, and compensatory over-inflation in the adjacent non-atelectatic lung;
- Pneumothorax: air in the pleural space with no vascular bed surrounding the visceral pleura;
- Bronchospasm: newly detected expiratory wheezing treated with bronchodilators;
- Aspiration pneumonitis: acute lung injury following the inhalation of regurgitated gastric contents.
The most common complications appear to be: pleural effusion (up to 95%), atelectasis (88%), prolonged mechanical ventilation (58%), diaphragmatic dysfunction (54%), and pneumonia (20%) (15). A recent review by Abbott et al. provided new recommendations regarding the definition of PPC, which will serve to standardize the outcomes of future studies and give a more homogeneous picture of pulmonary complication (16).
One-lung ventilation (OVL)
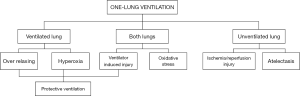
OVL is a widely used technique that relies on the use of double lumen endotracheal tubes to ventilate only one lung while the other is either collapsed by the surgeon or allowed to passively deflate. It is a crucial ventilation technique in thoracic surgery as it allows the removal of pathological tissue from one lung while maintaining an adequate level of oxygenation through to the other. Moreover, it can be used to improve the surgeon’s visibility of the operating field in other types of surgery. Despite this, the stop-ventilation of one lung creates a 50% right-to-left shunt, resulting in hypoxemia caused by ventilation/perfusion mismatch (17). However, shunt percentage is limited by pulmonary vasoconstriction secondary to hypoxemia. Other factors can affect this response: lateral positioning of the patient during surgery leads to an increase in gravity-dependent perfusion to the ventilated lung; surgical manipulation of the collapsed lung that obstructs blood flow to the other lung. The last important issue of this kind of surgery is that the collapsed lung is not properly drained from the lymphatic system, consequently leading to significant cytokine activation, while the other lung is often overextended by the ventilator with subsequent volutrauma (18-20).
All these factors involve an increased risk of lung injury in thoracic surgery with OLV, ranging from mild to severe acute respiratory distress syndrome (ARDS), and, consequently, an increase of mortality and morbidity (21).
State-of-the-art of mechanical ventilation in cardiothoracic surgery
Protective ventilation during cardiac surgery
For patients undergoing cardiac surgery, high tidal volumes (TV) (up to 10–12 mL/kg) are generally used to minimize atelectasis, and low levels of positive end-expiratory pressure (PEEP) are set to minimize hemodynamic impact. Based on the results of clinical studies in patients with ARDS, interest in the use of protective ventilatory strategies during heart surgery is growing.
Most of the data regarding protective ventilation come from experiences in intensive care and general surgery, advocating the use of medium/high TV and moderate PEEP (22); however, there is still no strong evidence in the context of cardiac surgery.
Currently, there is increasing evidence for the use of protective lung ventilation in patients undergoing general anesthesia in abdominal surgery (associated with a minor inflammatory response and best outcome). However, in cardiac and thoracic surgery, the application of a protective strategy is only associated with a reduced inflammatory response (23,24).
Protective ventilation should always be considered in lung disease, prolonged anesthesia, or surgical patients at high risk of postoperative complications. Although this strategy can be beneficial for the lungs, it can influence the cardiovascular system by reducing venous return and cardiac output, requiring fluids and vasopressors.
Two recent meta-analyses show how protective ventilatory strategies during general anesthesia can help reduce PPC and, sometimes, hospital stay (25,26).
Although the matter is still unclear, two recent papers have analyzed the state-of-the-art, and some recommendations can therefore be made regarding the ventilation strategy to adopt during cardiac surgery (27,28).
We must first separate the times that precede and follow CPB from the period during which the patient is under CPB. As regards the first, the recommendations made for mechanical ventilation in general anesthesia appear to be valid:
- Moderate TV [6–8 mL/kg of ideal body weight (IBW)] and moderate PEEP levels (2–5 cmH2O) (26);
- Use of recruitment maneuvers: although the studies agree on the usefulness of recruitment maneuvers, the best method has not yet been defined, nor the ideal pressure to be reached during the maneuver (29);
- Moderate hyperoxia (FiO2 not exceeding 80%) (30).
Regarding the management of CPB time, three options are available:
- Continuous-positive airway pressure (CPAP): various studies used CPAP with pressures between 5 and 15 cmH2O, showing different results;
- Mechanical ventilation: low tidal-low frequency ventilation showed a positive effect on secondary outcomes as postoperative oxygenation;
- Resting lung: this would seem to be the most comfortable option for the surgeon, but in the studies examined in the reviews there were no significant differences in surgical times compared to experimental arms.
None of the studies considered in the two reviews showed harm due to intra-CPB ventilation, nor due to pre- and post-CPB protective ventilation (27,31).
A 2015 review thoroughly investigated the use of oxygen.
Several studies reached the conclusion that moderate hyperoxia (0.5–0.8 FiO2) might help reduce ischemia reperfusion injury, as well as the incidence of surgical-site infections. However, not every study considered human subjects or cardiac surgery (32). A recent trial compared moderate hyperoxia with bypass surgery, aiming to detect myocardial damage. The use of a normoxemia strategy did not diminish myocardial damage, nor did it influence secondary outcomes such as cardiac index, systemic vascular resistance index, creatinine, and lactate (30). Another study, aiming to compare the creatinine increase in hyperoxygenated patients (1.0 FiO2) and those undergoing physiological oxygenation, is still underway (21).
A recent study focusing the postoperative period investigated whether an intensive alveolar recruitment strategy yields better results compared with a moderate strategy. All patients were ventilated with low-to-moderate TV. The intensive strategy (3 cycles of lung inflation (60 seconds each), consisting of a PEEP of 30 cmH2O, pressure-controlled ventilation, driving pressure of 15 cmH2O, respiratory rate of 15/min, inspiratory time of 1.5 seconds, and FiO2 of 0.40) was more able to prevent PPC, and reduce the incidence of severe pulmonary complications (29).
As for non-invasive ventilation (NIV), it can be used both to prevent and to treat PPC. This preemptive strategy does not seem to have produced significant results (33), while there is data in favor of NIV for the treatment of PPC. It seems that high-flow nasal cannulas are comparable with classic NIV (34).
Figure 1 summarizes issues and practices in mechanical ventilation during cardiac surgery.
Protective ventilation for OVL
In recent years, the number of respiratory complications caused by thoracic surgery and by OVL in general anesthesia have decreased thanks to the progress made in a type of ventilation considered to be more “protective” than the traditional method.
Currently, most authors support the fact that mechanical ventilation, especially OLV, can induce lung damage, measured both with local and systemic cytokine surveys (20,35,36). A study by Michelet et al. investigated plasmatic levels of interleukin (IL)-1beta, IL-6, IL-8, and tumor necrosis factor alpha in 52 patients undergoing planned esophagectomy for cancer. Patients were randomly assigned to a conventional ventilation strategy [TV =9 mL/kg during both two-lung ventilation (TLV) or OLV] or a protective ventilation strategy (TV =9 mL/kg during TLV reduced to 5 mL/kg during OLV). Pulmonary function and postoperative hospital stay were also evaluated. At the end of the trial, the protective ventilatory strategy led to a decrease in proinflammatory systemic response and an improvement of lung function (37).
Permissive hypercapnia can be caused by using a low TV. The use of a lower TV compared to traditional values, without increasing the respiratory rate, results in less pulmonary stress and less volume- or pressure-induced damage (37,38). This seems to be related to an increase in hypoxic pulmonary vasoconstriction, which consequently reduces the percentage of mechanically induced OLV shunt. Recent studies tend to confirm the potentially beneficial role of elevated levels of hypercapnia, since they seem to blunt the cytokine response (39).
A PEEP of 5 to 10 cmH2O is used in combination with low TV to prevent atelectasis and reduce lung injury secondary to mechanical stress caused by the ventilator. However, excessive PEEP can be harmful during OLV as it may cause a drop in perfusion of the ventilated lung leading to increased shunt (35).
To date, cytokine levels and lung histological analysis are in favor of using PEEP (40). Furthermore, a recent study by Ferrando et al. analyzed 30 patients undergoing thoracic surgery; randomly allocated into two arms, the first received a PEEP of 5 cmH2O, while the second was ventilated with an individualized PEEP level determined by a PEEP decrement trial (41). A single recruiting maneuver was carried out in both arms. At the end of the study, the patients ventilated with an individualized PEEP showed better results in terms of oxygenation and lung mechanics.
In general, patients should be ventilated with the lowest possible FiO2, keeping oxygen saturation above 90%, to avoid the production of reactive oxygen species (ROS) (42).
Oxygen toxicity during OLV is due to ischemia-reperfusion injury and oxidative stress. Collapsing the lung during surgery causes organ ischemia; when the lung is re-expanded, hypoxic vasoconstriction ends, permitting reperfusion and the release of ROS. The long duration of OLV leads to an increase in oxidative stress markers that after 120 minutes can be associated with a significant increase in respiratory failure and mortality (43). Furthermore, reducing lung exposure to oxygen can also have another benefit. We know that lungs exposed to 100% oxygen are more prone to atelectasis since oxygen is absorbed over time (a phenomenon known as “atelectasis by absorption”) (44).
It is now believed that, at the beginning of OLV, the use of a FiO2 of 0.8 might be appropriate. After about 20 minutes, when the maximum oxygenation value is reached and the lung has had sufficient time to adapt, FiO2 can be titrated down to the minimum value that permits a saturation greater than 90%. When pulmonary resection takes place, FiO2 can be further decreased since lung vessels are stopped, thus ending the phenomenon of pulmonary shunt (18,45,46).
Finally, two recent studies have compared protective ventilation strategies in OLV to conventional methods:
- A randomized trial of 100 patients undergoing thoracic surgery for lobectomy demonstrated that the incidence of pulmonary dysfunction (defined as PaO2/FiO2 <300 mmHg, lung infiltrates, or atelectasis) was significantly lower in patients ventilated with protective OLV (TV 5 mL/kg, PEEP 5 cmH2O, FiO2 0.5, pressure-controlled ventilator settings) compared with conventional OLV ventilation (TV 10 mL/kg, no PEEP, FiO2 1.0, volume-controlled ventilator settings) (47);
- An observational cohort study of 1,091 patients, before and after the implementation of a protective ventilation protocol during OLV (TV 5 mL/kg, use of PEEP and recruitment maneuvers, with limited maximal pressure ventilation), showed a lower incidence of acute lung injury and atelectasis, reduced admission rates to the intensive care unit, and a shorter length of hospital stay (48).
Figure 2 summarizes the open issues in OLV.
Conclusions
Ventilation management during cardiothoracic surgery is challenging. Although more and more studies seem to favor the use of protective ventilation, we are currently unable to propose guidelines on the correct management of intraoperative ventilation.
During the last decade, changes took place in the practice of pulmonary ventilation during cardiothoracic surgery, such as abandoning the use of high TV. Recent studies cited in this review show positive results on secondary or biochemical outcomes. Yet, there is no clear decrease in major outcomes, such as the incidence of severe pulmonary complication, mortality, or hospital stay.
However, four major trials are currently recruiting patients where postoperative pulmonary mortality and complications will be among the primary outcomes (31,49-51).
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Apostolakis E, Filos KS, Koletsis E, et al. Lung dysfunction following cardiopulmonary bypass. J Card Surg 2010;25:47-55. [Crossref] [PubMed]
- Barnas GM, Gilbert TB, Watson RJ, et al. Respiratory mechanics in the open chest: effects of parietal pleurae. Respir Physiol 1996;104:63-70. [Crossref] [PubMed]
- Locke TJ, Griffiths TL, Mould H, et al. Rib cage mechanics after median sternotomy. Thorax 1990;45:465-8. [Crossref] [PubMed]
- Gottschalk A, Cohen SP, Yang S, et al. Preventing and treating pain after thoracic surgery. Anesthesiology 2006;104:594-600. [Crossref] [PubMed]
- Jellish WS, Oftadeh M. Peripheral Nerve Injury in Cardiac Surgery. J Cardiothorac Vasc Anesth 2018;32:495-511. [Crossref] [PubMed]
- Dureja GP. Intercostal Neuralgia: A Review. J Neurol Transl Neurosci 2017;5:1076.
- DeFoe GR, Ross CS, Olmstead EM, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg 2001;71:769-76. [Crossref] [PubMed]
- Vlaar AP, Hofstra JJ, Determann RM, et al. Transfusion-related acute lung injury in cardiac surgery patients is characterized by pulmonary inflammation and coagulopathy: a prospective nested case-control study. Crit Care Med 2012;40:2813-20. [Crossref] [PubMed]
- Babik B, Asztalos T, Peták F, et al. Changes in respiratory mechanics during cardiac surgery. Anesth Analg 2003;96:1280-7. [Crossref] [PubMed]
- Schlensak C, Doenst T, Preuer S, et al. Cardiopulmonary bypass reduction of bronchial blood flow: A potential mechanism for lung injury in a neonatal pig model. J Thorac Cardiovasc Surg 2002;123:1199-205. [Crossref] [PubMed]
- Reber A, Budmiger B, Wenk M, et al. Inspired oxygen fraction after cardiopulmonary bypass: Effects on pulmonary function with regard to endothelin-1 concentrations and venous admixture. Br J Anaesth 2000;84:565-70. [Crossref] [PubMed]
- Müller H, Hügel W, Reifschneider HJ, et al. Lysosomal enzyme activity influenced by various types of respiration during extracorporeal circulation. Thorac Cardiovasc Surg 1989;37:65-71. [Crossref] [PubMed]
- Tassani P, Schad H, Schreiber C, et al. Extravasation of albumin after cardiopulmonary bypass in newborns. J Cardiothorac Vasc Anesth 2007;21:174-8. [Crossref] [PubMed]
- Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology 2010;113:1338-50. [Crossref] [PubMed]
- Wynne R, Botti M. Postoperative pulmonary dysfunction in adults after cardiac surgery with cardiopulmonary bypass: clinical significance and implications for practice. Am J Crit Care 2004;13:384-93. [PubMed]
- Abbott TE, Fowler AJ, Pelosi P, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth 2018;120:1066-79. [Crossref] [PubMed]
- Karzai W, Schwarzkopf K. Hypoxemia during one-lung ventilation prediction, prevention, and treatment. Anesthesiology 2009;110:1402-11. [Crossref] [PubMed]
- Lohser J, Slinger P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg 2015;121:302-18. [Crossref] [PubMed]
- Padley SP, Jordan SJ, Goldstraw P, et al. Asymmetric ARDS following pulmonary resection: CT findings initial observations. Radiology 2002;223:468-73. [Crossref] [PubMed]
- Gothard J. Lung injury after thoracic surgery and one-lung ventilation. Curr Opin Anaesthesiol 2006;19:5-10. [Crossref] [PubMed]
- Lopez MG, Pretorius M, Shotwell MS, et al. The Risk of Oxygen during Cardiac Surgery (ROCS) trial: study protocol for a randomized clinical trial. Trials 2017;18:295. [Crossref] [PubMed]
- Sutherasan Y, Vargas M, Pelosi P. Protective mechanical ventilation in the non-injured lung: review and meta-analysis. Crit Care 2014;18:211. [Crossref] [PubMed]
- Futier E, Constantin JM, Paugam-Burtz C, et al. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med 2013;369:428-37. [Crossref] [PubMed]
- Lellouche F, Dionne S, Simard S, et al. High tidal volumes in mechanically ventilated patients increase organ dysfunction after cardiac surgery. Anesthesiology 2012;116:1072-82. [Crossref] [PubMed]
- Yang D, Grant MC, Stone A, et al. A meta-analysis of intraoperative ventilation strategies to prevent pulmonary complications: is low tidal volume alone sufficient to protect healthy lungs? Ann Surg 2016;263:881-7. [Crossref] [PubMed]
- Hemmes SN, Neto AS, Schultz MJ. Intraoperative ventilatory strategies to prevent postoperative pulmonary complications: a meta-analysis. Curr Opin Anaesthesiol 2013;26:126-33. [Crossref] [PubMed]
- Bignami E, Guarnieri M, Saglietti F, et al. Mechanical Ventilation During Cardiopulmonary Bypass. J Cardiothorac Vasc Anesth 2016;30:1668-75. [Crossref] [PubMed]
- Garcia-Delgado M, Navarrete-Sanchez I, Colmenero M. Preventing and managing perioperative pulmonary complications following cardiac surgery. Curr Opin Anaesthesiol 2014;27:146-52. [Crossref] [PubMed]
- Costa Leme A, Hajjar LA, Volpe MS, et al. Effect of Intensive vs Moderate Alveolar Recruitment Strategies Added to Lung-Protective Ventilation on Postoperative Pulmonary Complications: A Randomized Clinical Trial. JAMA 2017;317:1422-32. [Crossref] [PubMed]
- Smit B, Smulders YM, de Waard MC, et al. Moderate hyperoxic versus near-physiological oxygen targets during and after coronary artery bypass surgery: a randomised controlled trial. Crit Care 2016;20:55. [Crossref] [PubMed]
- Lellouche F, Delorme M, Bussires J, et al. Perioperative ventilatory strategies in cardiac surgery. Best Pract Res Clin Anaesthesiol 2015;29:381-95. [Crossref] [PubMed]
- Ferrando C, Soro M, Belda FJ. Protection strategies during cardiopulmonary bypass: ventilation, anesthetics and oxygen. Curr Opin Anaesthesiol 2015;28:73-80. [Crossref] [PubMed]
- Pieczkoski SM, Margarites AG, Sbruzzi G. Noninvasive Ventilation During Immediate Postoperative Period in Cardiac Surgery Patients: Systematic Review and Meta-Analysis. Braz J Cardiovasc Surg 2017;32:301-11. [PubMed]
- Stéphan F, Barrucand B, Petit P, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA 2015;313:2331-9. [Crossref] [PubMed]
- Gajic O, Dara SI, Mendez JL, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med 2004;32:1817-24. [Crossref] [PubMed]
- Halbertsma FJ, Vaneker M, Scheffer GJ, et al. Cytokines and biotrauma in ventilator-induced lung injury: a critical review of the literature. Neth J Med 2005;63:382-92. [PubMed]
- Michelet P, D’Journo XB, Roch A, et al. Protective ventilation influences systemic inflammation after esophagectomy: a randomized controlled study. Anesthesiology 2006;105:911-9. [Crossref] [PubMed]
- Schilling T, Kozian A, Huth C, et al. The pulmonary immune effects of mechanical ventilation in patients undergoing thoracic surgery. Anesth Analg 2005;101:957-65. table of contents. [Crossref] [PubMed]
- Lang CJ, Barnett EK, Doyle IR. Stretch and CO2 modulate the inflammatory response of alveolar macrophages through independent changes in metabolic activity. Cytokine 2006;33:346-51. [Crossref] [PubMed]
- Ferreira HC, Mazzoli-Rocha F, Momesso DP, et al. On the crucial ventilatory setting adjustment from two- to one-lung ventilation. Respir Physiol Neurobiol 2011;179:198-204. [Crossref] [PubMed]
- Ferrando C, Mugarra A, Gutierrez A, et al. Setting individualized positive end-expiratory pressure level with a positive end-expiratory pressure decrement trial after a recruitment maneuver improves oxygenation and lung mechanics during one-lung ventilation. Anesth Analg 2014;118:657-65. [Crossref] [PubMed]
- Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care 2013;58:123-41. [Crossref] [PubMed]
- Misthos P, Katsaragakis S, Theodorou D, et al. The degree of oxidative stress is associated with major adverse effects after lung resection: a prospective study. Eur J Cardiothorac Surg 2006;29:591-5. [Crossref] [PubMed]
- Rothen HU, Sporre B, Engberg G, et al. Influence of gas composition on recurrence of atelectasis after a reexpansion maneuver during general anesthesia. Anesthesiology 1995;82:832-42. [Crossref] [PubMed]
- Karzai W, Klein U. FIO2 and studies on oxygenation during one-lung ventilation. Br J Anaesth 2012;109:644; author reply 644-5. [Crossref] [PubMed]
- Senturk M, Slinger P, Cohen E. Intraoperative mechanical ventilation strategies for one-lung ventilation. Best Pract Res Clin Anaesthesiol 2015;29:357-69. [Crossref] [PubMed]
- Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139:530-7. [Crossref] [PubMed]
- Licker M, Diaper J, Villiger Y, et al. Impact of intraoperative lung-protective interventions in patients undergoing lung cancer surgery. Crit Care 2009;13:R41. [Crossref] [PubMed]
- Bignami E, Guarnieri M, Saglietti F, et al. Different strategies for mechanical VENTilation during CardioPulmonary Bypass (CPBVENT 2014): study protocol for a randomized controlled trial. Trials 2017;18:264. [Crossref] [PubMed]
- . Available online: https://clinicaltrials.gov/ct2/show/NCT03372174Maintaining Mechanical Ventilation During Cardiopulmonary Bypass for Cardiac Surgery (VECAR).
- Nguyen LS, Merzoug M, Estagnasie P, et al. Low tidal volume mechanical ventilation against no ventilation during cardiopulmonary bypass heart surgery (MECANO): study protocol for a randomized controlled trial. Trials 2017;18:582. [Crossref] [PubMed]