Heart-Lung interaction in spontaneous breathing subjects: the basics
Introduction
The interaction of the heart and lungs is one of the basic rhythms of life and fluctuates with frequency of the heart beat and the frequency of breathing. The configuration of the circulation that exists in mammals and birds, in which blood flow in the pulmonary circulation passes between the right and left hearts, and is separated from that of the systemic circulation, began with amphibians (1). This organization had the advantage of allowing the pulmonary vascular pressure to be much lower pressure than the systemic vascular pressure, which in turn allowed alveola to be delicate structures with excellent gas exchange. However, because of its place in the mid-chest, and the requirement of blood flow to pass from the right to left heart through the inflating and deflating lungs, the heart became subject to the mechanical forces that inflate the lung. These consist primarily of two forces: changes in pleural pressure and changes in the pressure across the lung, which is called transpulmonary pressure (2,3). During spontaneous breathing, the fall in pleural pressure dominates the interaction by effectively lowering the heart relative to the rest of the body. This change in the environment of the heart relative to atmospheric pressure alters what comes back to the heart from the great veins and what goes out of the heart into the aorta. The second factor is the increase in transpulmonary pressure with lung inflation, and this component is the same for both spontaneous negative pressure breathing and positive pressure mechanical breaths. The increase in transpulmonary pressure can affect emptying of the right heart and filling of the left heart. I will begin with a summary of the major determinants of normal steady state cardiac output, independent of respiratory interactions with the heart. The principles in this review equally apply to the trigger phase of inspiration in someone with spontaneous efforts on a ventilator. The emphasis will be on Guyton’s analysis of the interaction of venous return and cardiac output (4) because his analysis is very useful for understanding the shifting relationship of the heart relative to the rest of the body.
Regulation of steady state cardiac output
To understand how breathing affects cardiac output, it first is necessary to understand the normal factors that regulate steady state cardiac output. Two functions determine cardiac output; one defines the return of blood to the heart and the other defines how well the heart handles the returning blood.
Return function
The vasculature consists of a series of elastic chambers and the properties of these chambers determine the flow of blood around the closed circuit that makes up the circulation (4-6). Volume filling the circulatory system stretches the elastic walls of all its components. Even when there is no flow, this creates a pressure that is the same in all vascular compartments and is called the mean circulatory filling pressure (MCFP) (7). Flow between compartments occurs when there is a pressure differences between the compartments. If a major vein is cut, even when there are no cardiac contractions, blood will drain from the circulation to atmospheric pressure until there is no longer stretch of the vascular walls. The determinants of flow in this simple situation are the volume that stretches the walls of all vascular compartments, the resistance draining the system to atmospheric pressure, and the compliance (1/elastance), or stretchiness of the vascular walls. The largest proportion of vascular volume is in venules and small veins, and thus this region dominates the elastic force returning blood to the heart. Because of its importance for cardiac filling it has a separate name, mean systemic filling pressure (MSFP). Under flow conditions volume redistributes among the vascular compartments and the pressure in the veins and venules is most often higher, but also can be lower than MCFP (2). The role of the heart in this conceptual model is to lower the downstream pressure, which is the right atrial pressure. This allows the venous volume to drain back to the heart. In most circumstances, right atrial pressure is equivalent to the central venous pressure (CVP) and the terms can be used interchangeably. The elastance of vascular structures is relatively constant, whereas the total vascular volume that stretches vessels can change, as can the resistances that connect each elastic region. The exceptions to this are the cardiac chambers. The elastance of the cardiac walls changes cyclically during the cardiac cycle. This transient increase in elastance during systole raises the pressure of the volumes contained in the ventricles. This raises the ventricular pressure relative to ventricular out-flows (pulmonary artery from the right and aorta from the left ventricle) and blood flows out the ventricles in one direction because of the presence of the cardiac valves. Each cycle produces a stroke volume that moves through the vasculature because of the pressure difference that is produce in each compartment compared to the next compartment. In the steady state, a stroke return to the right heart occurs that must be the same as the stroke volume ejected by the left heart. Only the equivalent of one stroke volume moves around the circuit in each cardiac cycle.
Cardiac function
As indicated in the previous section, cardiac chambers undergo cyclic changes in their elastance during the cardiac cycle. As described by the Frank-Starling relationship (8,9), the initial pressure in the ventricle, just before the onset of systole, is a critical determinant of the volume ejected per beat. This pressure is called the preload of the heart and it is determined by the volume in the ventricle at the end of diastole, and the diastolic elastance of the ventricle (10). The preload determines the final stretch of the cardiac sarcomeres before the onset of systole. The relationship of cardiac output to preload is called the cardiac function curve as defined by Starling (8). Other factors that determine the volume ejected per beat are the load the heart faces when it ejects, which is called the afterload, and the speed and extent of the transient increase in the elastance of the ventricles during the cardiac cycle, which is called contractility. The final determinant of how much the heart ejects per minute is the number of times per minute that the heart ejects a stroke volume, that is, heart rate. A decrease in afterload, an increase in contractility or an increase in heart rate shift the cardiac function curve upward and leftward. An increase in cardiac function is thus defined as an increase in cardiac output for the same preload. An increase in cardiac function needs to be distinguished from an increase in cardiac output. Function defines what the heart can do based on its filling pressure, whereas cardiac output indicates the volume leaving the heart per minute.
Cardiac output
The actual or “working” cardiac output is determined by the interaction of the venous return function, which determines how well the blood comes back to the heart, and the cardiac function curve, which determines how well the heart ejects the blood that comes back to it. The interaction of the return function and cardiac function is well described by Arthur Guyton’s graphical approach, which can be used to mathematically to solve the complicated interactions of these two functions (Figure 1). He plotted Pra on the x-axis because he argued that this is a major regulated variable for the return of blood to the heart. Right atrial pressure at the end of diastole also is the cardiac preload, which is the major variable in the cardiac function curve at a fixed afterload, heart rate and contractility. Guyton’s analysis is especially useful when interpreting how the heart interacts with the venous return during the ventilation cycle, because it allows one to appreciate how the reference system for the heart varies from that of the systemic circulation.
Effects of changes in pleural pressure
Flow into the right heart
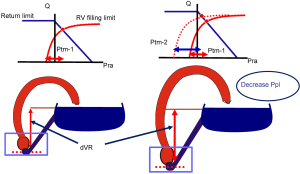
A key physiological principle is that the force stretching the wall of an elastic structure is the difference in pressure inside the elastic structure compared to the pressure outside the wall of the elastic structure. This is called transmural pressure and it is the force that determines the stretch of walls of the elastic structure. The heart is in the chest, and surrounded by pleural pressure which changes during the ventilation cycle. This means that the proper reference pressure for measuring the force across the wall of the heart is pleural pressure, which varies relative to an atmospheric reference during the ventilation cycle (11). Thus, a transducer outside the chest does not indicate what is happening to the transmural pressures of the heart. A simple way to think of what is happening is that on each spontaneous inspiration, the fall in pleural pressure effectively lowers the heart relative to the rest of the body. This means that on each inspiration the pressure difference for the return of blood to the heart increases, and accordingly right heart filling increases on inspiration. However, this is not obvious when looking at right atrial pressure which usually falls on inspiration, and makes it look like right heart filling is decreasing during inspiration (Figure 2). However, the fall in right atrial pressure occurs because the pressure is measured relative to atmospheric pressure rather than the pressure outside the heart, which is pleural pressure. When pleural pressure is measured, it can be seen that the pleural pressure falls more that the right atrial pressure, which means that transmural right atrial pressure actually increased during inspiration because its filling increased. The increase in right ventricular filling increases right ventricular stroke volume during inspiration. The volume then is passed to the pulmonary vasculature, and within a few beats, to the left heart in what is called a “series” effect. This results in an increase in left ventricular stroke volume which then increases arterial pressure during expiration at normal heart rates and breathing rates.
The change in filling of the heart during inspiration is easily seen on Guyton’s graphical analysis of the interaction of the venous return and cardiac functions (Figures 1,2). The venous circuit is surrounded by atmospheric pressure and is not altered by the fall in pleural pressure. However, the heart is surrounded by pleural pressure and not atmospheric pressure. At end-expiration (functional residual capacity), pleural pressure is negative relative to atmospheric pressure. Accordingly, when the heart is empty, the pressure inside the heart should also be negative relative to atmospheric pressure and the plot of the cardiac function curve starts from a negative. During a spontaneous inspiration the cardiac function curve must be shifted to the left of the venous return curve to account for the fall in pleural pressure. This results in the cardiac function curve intersecting the venous return curve at a higher cardiac output and lower right atrial pressure relative to atmospheric pressure, but with a higher transmural right atrial pressure.
Fluctuations in the filling of the left heart during ventilation likely are smaller than those on the right side with smaller increases after inspiration and consequently, less fall during late expiration, although this has not been quantitated well in vivo. The reason is that the volume in the pulmonary vasculature can “buffer” the changes in filling of the left heart because the pulmonary compliance can take up and release volume to the left heart independent of the stroke volume from the right ventricle (2).
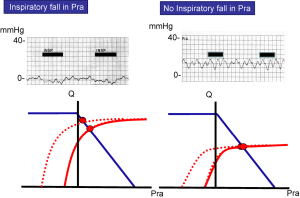
There are important limits to the inspiratory increase in right heart filling. Veins have floppy walls and when the pressure inside a floppy vein is less than the pressure outside the vein, it collapses. This does not stop flow, but limits a further increase in flow from a decrease in right atrial pressure below the collapse point in what is called a vascular waterfall (12). This is represented by the flat part of the venous return curve in Figure 1. When breathing spontaneously, the pressure outside the great veins, just as they enter the thorax, is atmospheric pressure. This means that when the pressure in the right heart drops below atmospheric pressure with the fall in pleural pressure, a further lowering of pleural pressure will not increase right heart filling. Right atrial pressure in a healthy upright resting human normally is below zero, which means that there is no inspiratory increase in right heart filling in this position.
The other important factor limiting the inspiratory increase in right heart filling is the volume capacity of the right ventricle. Right ventricular volume usually is limited by the pericardium (13), but even when there is no pericardium, the cardiac cytoskeleton limits cardiac filling. This is seen as a sharp break in the cardiac function curve (Figures 1,2) and when the limit is reached, increasing the venous return function increases only the pressure in the right heart, and not ventricular volume. There thus is no increase sarcomere stretch, and no change in stroke volume. In an average sized male the limit of right heart filling is 140 to 160 mL (14).
Although the right atrial pressure does not fall relative to atmospheric pressure during inspiration when the right heart is volume limited, right atrial transmural pressure increases significantly. We previously used this physiological point to predict fluid responsive in patients (10,15) (Figure 2). We reasoned that patients who have an inspiratory fall in right atrial pressure are functioning on the ascending part of the cardiac function curve and could respond to fluids, but not always, because they could be close to the plateau of the cardiac function curve. On the other hand, patients who have no inspiratory fall in right atrial pressure must be functioning on the flat part of the cardiac function curve, and should not respond to fluids. The test should be true whether the subject is intubated or not as long as the inspiratory effort is strong enough to create a sufficient fall in pleural pressure. We found that this was indeed true, although mainly in the negative sense; lack of a fall in right atrial pressure indicates that the patient will not respond to fluid. The same principle explains why a lack of decrease in the diameter of superior or inferior vena cava with spontaneous breaths indicates that the subject likely will not be fluid responsive (16-18). When right heart filling is limited, inspiration cannot increase the emptying of the large veins.
Flow out of the left heart
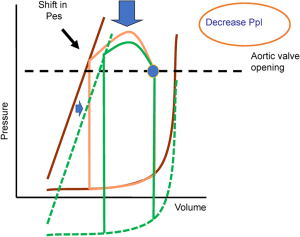
Arterial pressure falls on inspiration for two reasons. The first simply is that the decrease in pleural pressure is transmitted to the arterial pressure. However, not only does the pressure fall, but pulse pressure falls too, which indicates that stroke volume decreased. This decrease in stroke volume occurs because the effective lowering of the heart relative to the rest of the body means that the left heart must generate greater pressure to open the aortic valve and to reach the same arterial pressure relative to atmospheric pressure. This increases left ventricular afterload. The effect is greatest when peak inspiration occurs just as the aortic valve opens. The magnitude of the effect depends upon when in the cardiac cycle inspiration occurs (Figure 3). In subsequent beats, the retained volume in the left ventricle helps restore the stroke volume, and the effect usually only lasts for a few beats given the short time of inspiration. For example, at 10 breaths per minute, each breath is 6 seconds and inspiration is about 2 seconds. At a heart rate of 75 b/min, each heart beat is 0.8 seconds so that only 2 to 3 beats are affected, and even less at slower heart rates. The afterload increase is not much of a load for a normal left ventricular, but it can become a problem when left ventricular systolic function is depressed, or when the inspiratory fall in pleural pressure is very large and sustained. Repetitive and large negative efforts have been shown to produce pulmonary edema because of the increased afterload on the left heart combined with increase right heart filling. When this occurs, the pulmonary edema can be treated by relieving the airway obstruction (19). The effect on the left ventricular diastolic pressure can be greater than the effect on stroke volume when the left ventricle is operating on the steep part of its passive filling curve as is shown below during a Mueller maneuver (20).
Lung inflation
Right ventricular outflow
The effect of lung inflation on the heart is primarily related to the increase in transpulmonary pressure (21,22), although there is some direct compression of the heart by the lungs (23). It is commonly stated that lung inflation increases pulmonary vascular resistance by compressing pulmonary vessels and narrowing their diameter (24). This was primarily based on a paper in which only changes in pulmonary artery pressure were measured without considering the downstream pressure, which was assumed to be left atrial pressure or even zero (25). When the downstream pressure was accounted for by obtaining a number of points on a pulmonary flow-pressure line, it because apparent that lung inflation does not have a large effect on pulmonary vascular resistance (21). This should have been predicted because blood is not easily compressible. What was found was that a sufficiently large increase in transpulmonary pressure creates a pressure on the outside of intrapulmonary vessels that is greater than the pressure inside them and these vessels develop West zone II conditions, or vascular waterfalls, and flow limitation (21,26). This can happen because the right ventricle is surrounded by pleural pressure and decreases with the decrease in pleural pressure relative to atmospheric pressure (Figure 4). There is also a decrease in pressure from the main pulmonary artery to the vessels between alveolar because of the resistance along the vessel. When these factors are of sufficient magnitude, West zone II and even I conditions are produced, and alveolar pressure becomes the downstream pressure for pulmonary flow, and the load on the right ventricle (21,27). This is easier to understand with positive pressure breathing and more difficult with spontaneous breathing but still can occur if a fall in pleural pressure decreases the pulmonary pressure sufficiently below alveolar pressure (Figure 4).
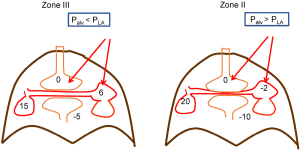
Lung inflation also increases filling of the left heart. There are two kinds of vessels in the lungs; inter-alveolar vessels, which are between alveolar, and intra-alveolar vessels, which are in the corners between alveolar (22). The blood in the inter-alveolar region can be squeezed out by the inflating lung and increase left heart filling, whereas the intra-alveolar vessels can be stretched and take up volume. However, the increase in intra-alveolar vessels usually is maximal at a pulmonary venous pressure of about 3 mmHg, which is at the low end of normal left atrial pressure. Thus, the usual dominant effect of lung inflation is an inspiratory increase in left heart filling of about 2 mmHg. This adds to the increase in left heart volume that is caused by the inspiratory increase in afterload on left heart as discussed in the last section.
As an example of this, we previously showed that the peak change in pulmonary artery occlusion pressure during a spontaneous inspiratory effort closely matches the fall in esophageal pressure, and thus can track inspiratory efforts relative to atmosphere (28,29). However, the fall in pulmonary artery occlusions pressure was on average 2 mmHg less than the fall in esophageal pressure because of the inspiratory increase in left heart filling.
Abdominal component
Expiration normally is passive and CVP does not change during expiration. However, abdominal muscles can be activated during expiration and increase abdominal pressure (29,30). This can increase the return of blood to the right heart from the abdominal compartment and produce an expiratory component to right heart filling. This is the same as occurs with a hepato-jugular reflux (31). If right heart volume is limited, the increase in abdominal pressure produces a respiratory increase in right heart pressure but no change in outflow from the right ventricle, which is called Kussmaul’s sign (31,32).
Neuromodulation
Changes in lung volume can produce changes in heart rate by activation of afferent signals by the lung and reduction of vagal tone. These increase heart rate during inspiration and slow the heart rate during expiration in what is called sinus arrhythmia. This pattern is more common in young individuals. As indicated above, the changes in heart rate will modify the effects of changes in pleural pressure and lung inflation. An increase in heart rate during inspiration would help the heart handle the normal increase in venous return associated with a spontaneous inspiration (33).
Respiratory maneuvers
Mueller maneuver
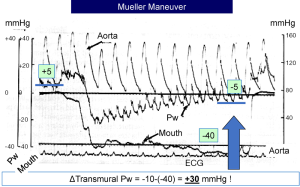
This is an inspiration against a closed glottis. Because the glottis is closed, there is no change in lung volume. Thus, this maneuver allows an assessment of the pure effect of a decrease in pleural pressure without a change in transpulmonary pressure and lung volume (20). An example is shown in Figure 5 in which the negative inspiratory pressure was held at −40 mmHg. The decrease in pleural pressure produced a sustained increase in left ventricular afterload and at the same time, increased right heart filling. The arterial pressure was maintained after the first beat, but there was an initial fall in left atrial pressure (based on the pulmonary artery occlusion pressure). The left atrial pressure then progressively increased while the negative pleural pressure was maintained. This indicates that there was a marked increase in transmural left atrial pressure. In this example, the transmural left atrial pressure increased by 30 mmHg based on the change in pleural pressure of −40 mmHg and the change in left atrial pressure of −10 mmHg. If an X-ray had been performed, it would look like the person in pulmonary edema. Note the large increase in the ‘v’ wave on the pulmonary artery occlusion tracing at the end of the maneuver, which indicates a decrease in the diastolic compliance of the left heart due to the increased filling.
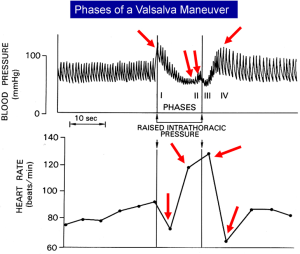
Valsalva maneuver
The opposite of a Mueller maneuver is a Valsalva maneuver, which is an expiration against a closed glottis. This maneuver produces a marked rise in pleural pressure without a change in lung volume and results in changes in blood pressure and heart rate (34). An example is shown in Figure 6. At the beginning of the maneuver, arterial pressure rises because the increased pleural pressure is transmitted to the heart, and increases left ventricular and arterial pressures, but this only lasts for a few beats. The rise in pleural pressure inhibits venous return and thus cardiac output, and blood pressure fall. As a consequence, baroreceptors in the carotid sinus are activated. Vagal tone is decreased and sympathetic activity increased, which increases heart rate and peripheral vascular resistance in an attempt to restore the arterial pressure. When the Valsalva is stopped, the decrease in pleural pressure initially results in a further drop in arterial pressure, which triggers a further rise in heart rate, but then the backed up venous volume increases venous return, cardiac output and arterial pressure rise, and vagal tone again increases to and slow the heart rate. These reflex changes in heart rate and blood pressure do not occur in the Mueller maneuver because the arterial pressure is relatively maintained.
Conclusions
The dominant effect of a spontaneous inspiratory effort is production of a transient increase in the filling of the right heart, but this response depends upon the potential for venous return to increase, and the capacity of the right ventricle to take up more volume. This process is best understood by appreciating that changes in pleural pressure with inspiration change the environment of the heart relative to the rest of the body. Other effects of spontaneous inspiratory efforts include transiently increased afterload on the left ventricle, inspiratory increase in left heart filling, possible increased loading of the right ventricle, and neuro-humeral responses, but these usually only are significant when pathological conditions are present.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Bishopric NH. Evolution of the heart from bacteria to man. Ann N Y Acad Sci 2005;1047:13-29. [Crossref] [PubMed]
- Magder S, Guerard B. Heart-lung interactions and pulmonary buffering: Lessons from a computational modeling study. Respir Physiol Neurobiol 2012;182:60-70. [Crossref] [PubMed]
- Magder S. Mechanical interactions between the respiratory and circulatory systems. In: Bradley TD, Floras JS. editors. Sleep Apnea: implications in cardiovascular and cerebrovascular disease. Lung Biology in Health and Disease. New York: Informa Health Care USA, Inc., 2010:40-60.
- Guyton AC. Determination of cardiac output by equating venous return curves with cardiac response curves. Physiol Rev 1955;35:123-9. [Crossref] [PubMed]
- Magder S. An Approach to Hemodynamic Monitoring: Guyton at the Beside. Critical Care 2012;16:236-43. [Crossref] [PubMed]
- Guyton AC, Lindsey AW, Bernathy B, et al. Venous return at various right atrial pressures and the normal venous return curve. Am J Physiol 1957;189:609-15. [Crossref] [PubMed]
- Guyton AC, Polizo D, Armstrong GG. Mean circulatory filling pressure measured immediately after cessation of heart pumping. Am J Physiol 1954;179:261-7. [Crossref] [PubMed]
- Starling EH. The Linacre Lecture of the Law of the Heart. London: Longmans, Green & Co., 1918.
- Katz AM. Ernest Henry Starling, His Predecessors, and the “Law of the Heart”. Circulation 2002;106:2986-92. [Crossref] [PubMed]
- Magder S. Right Atrial Pressure in the Critically Ill: How to Measure, What Is the Value, What Are the Limitations? Chest 2017;151:908-16. [Crossref] [PubMed]
- Magder S. Diagnostic Information from the Respiratory Variations in Central Hemodynamics Pressures. In: Scharf SM, Pinsky MR, Magder S. editors. Respiratory-Circulatory Interactions in Health and Disease. New York: Marcel Dekker, Inc., 2001:861-82.
- Permutt S, Riley S. Hemodynamics of collapsible vessels with tone: the vascular waterfall. J Appl Physiol 1963;18:924-32. [Crossref] [PubMed]
- Holt JP, Rhode EA, Kines H. Pericardial and ventricular pressure. Circulation Research 1960;8:1171-81. [Crossref] [PubMed]
- Cain PA, Ahl R, Hedstrom E, et al. Age and gender specific normal values of left ventricular mass, volume and function for gradient echo magnetic resonance imaging: a cross sectional study. BMC Med Imaging 2009;9:2. [Crossref] [PubMed]
- Magder SA, Georgiadis G, Cheong T. Respiratory variations in right atrial pressure predict response to fluid challenge. J Crit Care 1992;7:76-85. [Crossref]
- Airapetian N, Maizel J, Alyamani O, et al. Does inferior vena cava respiratory variability predict fluid responsiveness in spontaneously breathing patients? Crit Care 2015;19:400. [Crossref] [PubMed]
- Slama M, Masson H, Teboul JL, et al. Respiratory variations of aortic VTI: a new index of hypovolemia and fluid responsiveness. Am J Physiol Heart Circ Physiol 2002;283:H1729-H33. [Crossref] [PubMed]
- Vignon P, Repesse X, Begot E, et al. Comparison of Echocardiographic Indices Used to Predict Fluid Responsiveness in Ventilated Patients. Am J Respir Crit Care Med 2017;195:1022-32. [Crossref] [PubMed]
- Timby J, Reed C, Zeilender S, et al. “Mechanical” causes of pulmonary edema. Chest 1990;98:973-9. [Crossref] [PubMed]
- Magder SA, Lichtenstein S, Adelman AG. Effects of negative pleural pressure on left ventricular hemodynamics. Am J Cardiol 1983;52:588-93. [Crossref] [PubMed]
- Permutt S, Bromberger-Barnea B, Bane HN. Alveolar pressure, pulmonary venous pressure, and the vascular waterfall. Med Thorac 1962;19:239-60. [PubMed]
- Howell JB, Permutt S, Proctor DF, et al. Effect of inflation of the lung on different parts of pulmonary vascular bed. J Appl Physiol 1961;16:71-6. [PubMed]
- Butler J. The heart is in good hands. Circulation 1983;67:1163-8. [Crossref] [PubMed]
- Nunn J. Nunn’s Applied Respiratory Physiology. In: Lumb ABP, Ronald G. editor. Nunn’s Applied Respiratory Physiology. 7 edition. New York: Elsevier, 2010:99-117.
- Whittenberger JL, McGregor M, Berglund E, et al. Influence of state of inflation of the lung on pulmonary vascular resistance. J Appl Physiol 1960;15:878. [Crossref] [PubMed]
- West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lungs: relation to vascular and alveolar pressures. J Appl Physiol 1964;19:713-24. [Crossref] [PubMed]
- Lopez-Muniz R, Stephens NL, Bromberger-Barnea B, et al. Critical closure of pulmonary vessels analyzed in terms of Starling resistor model. J Appl Physiol 1968;24:625-35. [Crossref] [PubMed]
- Bellemare P, Goldberg P, Magder S. Variations in pulmonary artery occlusion pressure to estimate changes in pleural pressure. Intensive Care Med 2007;33:2004-8. [Crossref] [PubMed]
- Verscheure S, Massion PB, Gottfried S, et al. Measurement of pleural pressure swings with a fluid-filled esophageal catheter vs pulmonary artery occlusion pressure. J Crit Care 2017;37:65-71. [Crossref] [PubMed]
- Magder S, Serri K, Verscheure S, et al. Active Expiration and the Measurement of Central Venous Pressure. J Intensive Care Med 2018;33:430-5. [Crossref] [PubMed]
- Ducas J, Magder SA, McGregor M. Validity of the hepato-jugular reflux as a clinical test for congestive heart failure. Am J Cardiol 1983;52:1299-303. [Crossref] [PubMed]
- Takata M, Beloucif S, Shimada M, et al. Superior and inferior vena caval flows during respiration: pathogenesis of Kussmaul's sign. Am J Physiol 1992;262:H763-70. [PubMed]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biol Psychol 2007;74:263-85. [Crossref] [PubMed]
- Smith JJ, John P. Circulatory Response to Nonexercise Stress. Circulatory Physiology-the essentials. Baltimore: Williams and Wilkins, 1984:246-64.