The role of dobutamine stress echocardiography based projected aortic valve area in assessing patients with classical low-flow low-gradient aortic stenosis
Aortic stenosis (AS) is the most common valvular heart disease in the US (1). The prevalence of AS increases with age from 0.2% in those aged 50–59 years to 2.8% in adults older than 75 years and up to 9.8% in octogenarians (2,3). It is estimated that AS is responsible for yearly 85,000 aortic valve replacement and 15,000 deaths in North America (4).
Multiple echocardiography-based values including trans-valvular peak velocity, mean trans-aortic gradient, and aortic valve area (AVA) assessed by the continuity equation are essential in the diagnosis and severity assessment of AS.
AS is considered severe when the following criteria are met. First, either AVA is less than 1.0 cm2 or indexed AVA is less than 0.6 cm2/m2. Second, trans-valvular peak velocity is more than 4 m/s, and/or trans-valvular mean aortic valve gradient is more than 40 mmHg. Unfortunately, around 40% of AS patients meet one of the two criteria with most common scenario of having AVA <1.0 cm2 associated with low trans-valvular mean gradient (MG) of <40 mmHg and trans-valvular peak velocity of <4 m/s (5). This combination is called low-gradient AS (LGAS), which emerged as a challenging entity of AS. To discriminate this group more accurately the concept of transaortic flow was introduced using the stroke volume index (SVI). Those who has SVI of <35 mL/m2 were called low flow (LF), and those with SVI ≥35 mL/m2 were called normal flow (NF) (5). Therefore, LG AS patients with SVI of <35 mL/m2 were called LFLG AS; while LG AS with SVI ≥35 mL/m2 were called NFLG AS.
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines defined all symptomatic AS patient as staged D. Patients who had MG of more than 40 mmHg were classified as D1 regardless of the ejection fraction (EF) or flow (5). The guidelines recognized that two additional groups of LFLG AS and separated them depending on the EF to two separate groups. Patient with LFLG AS and reduced EF of less than 50% were defined as classical LFLG AS or D2. Those with LFLG AS and preserved EF (EF ≥50%) were defined as “paradoxical” LFLG AS or D3 (5). The ACC/AHA Guidelines failed to recognized patients with NFLG AS. The Heart Valve Clinic International database group identified those with NFLG AS and EF ≥ as D4 group (6).
Dobutamine stress echocardiography (DSE) is instrumental in determining whether patients with AVA <1.0 cm2 and LFLG pattern truly have severe AS. True-severe AS (TS-AS) is defined if either the MG is ≥40 mmHg or peak velocity is ≥4 m/s at maximum dobutamine dose. While pseudo severe AS (PS-AS) is recognized if the MG remains <40 mmHg and the peak velocity remains <4 m/s (5). Unfortunately, the validity of DSE is limited in patient with reduced LV flow defined as less than 20% increase in SVI at maximum dobutamine dose. Figure 1 details the different groups of LGAS.
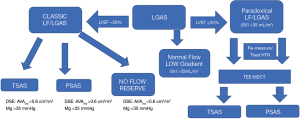
Annabi et al. (7) proposes that projected AVA (AVAproj) assessed at a NF status, may offer a better discrimination tool to detect TS-AS. AVAproj is a concept that was introduced by Clavel et al. in 2010 as a better predictor of outcomes in medically treated AS patients when compared to DSE (4). AVAproj is using the following equation:
AVAproj = AVArest + [(AVApeak − AVArest)/(Qpeak − Qrest)] × (250 − Qrest)
AVA was calculated using this equation:
AVA = [3.14 × (LVOT/2)2 × V1]/V2
V1 = LVOT velocity (m/s); V2 = maximum transvalvular velocity (m/s); LVOT = left ventricular outflow track diameter (cm); Q = SV/LVET; SV = stroke volume; LVET = left ventricular ejection time.
AVArest is AVA at rest, AVApeak is AVA assessed at peak stress (defined as the time when MG is maximum during DSE). Qrest is assessed at baseline while Qpeak is assessed at peak stress.
Annabi et al. (7) excluded patients with reduced flow reserve rate (around 26%). TS AS patients had smaller AVAproj (0.88±0.16 vs. 0.99±0.23 cm2; P<0.01) and smaller AVAproj indexed [AVA divided by body surface area (BSA)] (0.45±0.07 vs. 0.54±0.14 cm2/m2; P<0.0005) when compared to those with PS-AS. Furthermore, Annabi et al. (7) suggests that that AVAproj was more predictive of severity of AS (70%) than MG at peak stress (48%), peak stress AVA (60%) or the combination of both (47%) (7). It also suggests that lowering the peak stress MG cutoff to 35 mmHg increased the sensitivity of MG in identifying TS-AS. In patients who were managed medically, AVAproj was predictive of mortality, while peak stress MG and peak AVA were not after adjustment for age, sex, functional capacity, kidney disease, and EF at peak stress. Although it should be noticed that only few patients with peak MG >40 were managed medically in this study.
There are few limitations to the finding suggested by Annabi et al. (7). The most important one is the imitation in AVAproj which requires the measurement of multiple variables including LVOT diameter, LVOT velocity, aortic valve velocity, and LV ejection time at rest and during dobutamine stress. Each of these measurements can be vulnerable to measurement variability that can affect the validity of the calculation. Also the study excluded patients with reduced LV flow reserve, so it did not provide a solution for this patient population. Nonetheless, the study shed some light on the possible imitations of the current standard use of DSE in patient with reasonable.
In patients with LF/LGAS and with reduced LV flow reserve, TOPAS study recommended to use aortic valve calcium scoring by computed tomography to corroborate stenosis severity. A calcium score value of ≥1,274 AU in women and ≥2,065 AU in men were determined to be reasonable cutoff values to confirm severe AS (8-10). However, while several studies showed that aortic valve calcium score was strongly associated with the severity of AS and clinical outcomes (8-10); these calcium score thresholds were never validated in D2 patients (8-10). Some have suggested that the use of dobutamine with pressure wire may provide an efficient and safe alternative to assess the severity of AS in LFLG and NFLG AS without the potential impact of LVOT measurement errors on AS assessment (11). However, Cath lab based assessment of AS might be also affected by many pitfalls related to Cath lab based cardiac output assessment (12-14).
In summary, while DSE based MG is currently recommended by the ACC/AHA guidelines, it seems in reality there is no real gold-standard for determining AS severity in D2 patients especial in the subset of patients with reduced LV flow reserve. While DSE and Cath lab measurements with dobutamine are used by many labs; the use of AVAproj and AVAproj indexed with or without MG peak values is a new method that may help determine the severity of AS. Further studies comparing these different modalities may be needed in the future to better define which patients may benefit the most of surgical and/or transcatheter intervention.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Eveborn GW, Schirmer H, Heggelund G, et al. The evolving epidemiology of valvular aortic stenosis. the Tromso study. Heart 2013;99:396-400. [Crossref] [PubMed]
- Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. J Am Coll Cardiol 1997;29:630-4. [Crossref] [PubMed]
- Clavel MA, Burwash IG, Pibarot P. Cardiac Imaging for Assessing Low-Gradient Severe Aortic Stenosis. JACC Cardiovasc Imaging 2017;10:185-202. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438-88. [Crossref] [PubMed]
- Dulgheru R, Pibarot P, Sengupta PP, et al. Multimodality Imaging Strategies for the Assessment of Aortic Stenosis: Viewpoint of the Heart Valve Clinic International Database (HAVEC) Group. Circ Cardiovasc Imaging 2016;9. [Crossref] [PubMed]
- Annabi MS, Touboul E, Dahou A, et al. Dobutamine Stress Echocardiography for Management of Low-Flow, Low-Gradient Aortic Stenosis. J Am Coll Cardiol 2018;71:475-85. [Crossref] [PubMed]
- Clavel MA, Messika-Zeitoun D, Pibarot P, et al. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study. J Am Coll Cardiol 2013;62:2329-38. [Crossref] [PubMed]
- Clavel MA, Pibarot P, Messika-Zeitoun D, et al. Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol 2014;64:1202-13. [Crossref] [PubMed]
- Aggarwal SR, Clavel MA, Messika-Zeitoun D, et al. Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging 2013;6:40-7. [Crossref] [PubMed]
- Fanari Z, Gunasekaran PC, Shaukat A, et al. Safety and utility of dobutamine and pressure wire use in the hemodynamic assessment of low flow, low gradient aortic stenosis with reduced left ventricular ejection fraction. Cardiovasc Revasc Med 2018;19:438-43. [PubMed]
- Fanari Z, Grove M, Rajamanickam A, et al. The Impact of Direct Cardiac Output Determination on Using A Widely Available Direct Continuous Oxygen Consumption Measuring Device on The Hemodynamic Assessment of Aortic Valve. Del Med J 2016;88:270-5. [PubMed]
- Fanari Z, Rajamanickam A, Grove M, et al. Impact of Catheterization Lab Computer Software Settings on Hemodynamic Assessment of Aortic Stenosis. Del Med J 2016;88:212-7. [PubMed]
- Fanari Z, Grove M, Rajamanickam A, et al. Cardiac output determination using a widely available direct continuous oxygen consumption measuring device: a practical way to get back to the gold standard. Cardiovasc Revasc Med 2016;17:256-61. [Crossref] [PubMed]


