DUSP2 methylation is a candidate biomarker of outcome in head and neck cancer
Introduction
Despite improving clinical outcomes, optimal protocols for administration of combined modality therapy and biomarkers to inform the optimal use of such therapy have not been definitively identified in head and neck squamous cell carcinoma (HNSCC) (1). We have previously shown that mutations and polymorphisms in TP53 and in MDM2 predict response and survival in HNSCC patients treated with platinum-based chemoradiotherapy (CRT) (2-4).
The dual specificity phosphatases (DUSPs) comprise a large family of genes encoding enzymes which catalyse the dephosphorylation of serine, threonine and tyrosine residues in different types of mitogen-activated protein kinases within the MAPK TXY (Thr-Xaa-Tyr) motif (5). DUSP2 is an inducible nuclear phosphatase highly expressed in activated immune cells (6,7).
DUSP2 acts as a tumour suppressor at least in part via its physiological substrate ERK2 (8,9). Phosphorylation of ERK1/2, via Ras/Mek/ERK pathway, activates cell proliferation and survival (10-13) in response to a wide range of stimuli, including radiation, hypoxia and chemotherapeutic agents (14).
Silencing of DUSP2 leads to prolonged activation of the ERK pathway (15) that in turn has been associated with many aspects of tumour phenotype (16-18). Nonetheless DUSP2 has been involved in the initiation and development of acute leukaemia (19) and in the pathogenesis of human solid cancers, where a loss of its expression has been associated with breast, colon, lung, ovary, kidney and prostate tumours in hypoxic conditions (8,15). The strong relationship between hypoxia and downregulation of DUSP2 affords a mechanistic link with angiogenesis and metastasis (20).
Overexpression of EGFR, leading to proliferation, angiogenesis and metastasis is frequently observed in HNSCC (21), but correlation with hypoxia is still debated (22,23). DUSP2 affects the sensitivity of cancer cells to chemotherapy in vitro (15). Moreover, DUSP2 is a transcriptional target of wild-type p53 (24) a determinant of treatment response in HNSCC (25).
In the present study, we have tested the hypothesis that DUSP2 is transcriptionally silenced by methylation in HNSCC and in turn investigated the role of DUSP2 as determinant of clinical outcome in locally advanced head and neck squamous cell carcinoma (LA-HNSCC) treated with platinum-based CRT.
Methods
Cell lines
Five human HN cancer cell lines (CAL27, CAL33, HEp-2, HNO41, HNO91) were used to evaluate DUSP2 expression and aberrant methylation in the CpG island. Cells were routinely cultured as previously described (26).
Gene expression analysis
Real-time qRT-PCR was performed on RNA extracted from cell lines using the RNeasy Mini Kit (Qiagen, Crawley, West Sussex, UK) according to standard procedures. DUSP2 expression was assessed by TaqMan PCR assay Hs00358879_m1 using the ABI-Prism 7000 Sequence Detection System (Life Technologies, Carlsbad, California, US). Beta-2-microglobulin (Hs99999907_m1) was used as a control housekeeping gene.
Clinical cases and molecular analyses
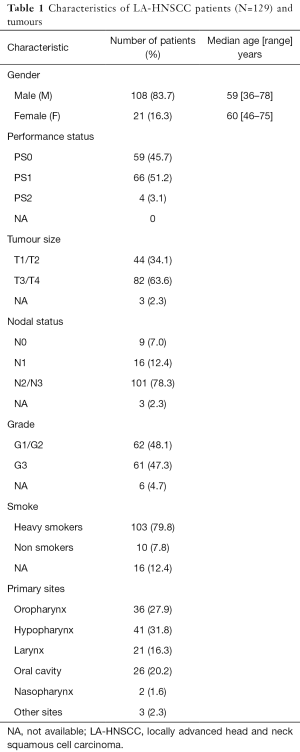
Formalin fixed paraffin embedded (FFPE) specimens were obtained at diagnosis from 129 LA- HNSCC patients (stage III and IV) in Cuneo, from 1998 to 2013.
The distribution of gender, age, performance status (PS), tumor size (T), lymph nodes (N), histological grade (G), smoking habit and primary sites is shown in Table 1.
Full table
Smokers were defined according to Ang et al. [2010] (27) with a cut-off of 10 pack-year.
Mutational analysis of TP53 and genotyping of the SNP Pro72Arg (rs1042522) were performed as previously described (2-4). TP53 mutations were classified according to the IARC TP53 mutation database (http://wwwp53.iarc.fr/), the Poeta et al. [2007] (28) study and the Evolutionary Action score of TP53 (EAp53) (http://mammoth.bcm.tmc.edu/EAp53/) (29). EGFR was quantified in 72 patients by IHC as already described (30).
HPV was searched by DNA-PCR using specific primer pairs for type 16 in 129 patients’ tissues; IHC staining for p16 (31) was performed in 118 patients’ tissues. This work was carried out in accordance with the Declaration of Helsinki. The study and the informed consent for biological samples collection and research proposal, obtained from patients, were approved by the Ethical Committee of S. Croce & Carle Teaching Hospital in Cuneo (approval n. 198/13).
Methylation analysis
DNA samples were extracted from cell lines and FFPE tissues using standard protocols. In particular, genomic DNA was purified by proteinase K digestion of 10 m sticks cut from paraffin sections using xylene–phenol protocol, as previously described (3).
DUSP2 CpG island methylation was analyzed by pyrosequencing (Biotage, Uppsala, Sweden). Following PCR primers were designed to amplify a fragment of 134 bp, covering part of IVS I and exon 2: F 5'-GTAGATAGGAGTTTTGGAGT-3'; R5'-BIOT-CTCTTCCCCTCCTTACAAA-3'. 500 ng genomic DNA were amplified and analysed by QCpG Software (Qiagen) as already reported (32).
We used a universal commercial human DNA (CpGenome Universal Methylated DNA, Millipore Corporation, Billerica, MA, USA) as methylated (met) control (average methylation 98%), while DNA obtained from a pool of 5 healthy head and neck epithelia was employed as unmethylated (unmet) control [mean value of DUSP2 CpG island methylation was 4% (range, 3–7%)]. On the basis of this consideration we established a methylation cut-off of 10%.
Statistical analysis
Relationships between DUSP2 gene methylation, clinical (gender, tumour site, PS, T, N, G, smoke and clinical response) and molecular (SNP rs1042522 and TP53 sequence) characteristics were analysed by cross-tabulation.
OS analysis was based on the time from diagnosis to death or last contact in which the survivors were censored. PFS analysis was based on the time from diagnosis to first event (loco-regional recurrence or distant metastasis); patients without an event were censored at their last follow-up.
OS and PFS curves were calculated using the Kaplan-Meier method, where statistical significance of each variable was tested with log-rank test.
Univariate analysis was performed on each variable, then for DUSP2/EGFR (N=72) and DUSP2/TP53 (N=101), and lastly in 70 DUSP2/EGFR/TP53 patients’ combination.
Significant variables identified in the univariate analysis with P≤0.05 entered into a multivariate analysis performed on 70 DUSP2/EGFR/TP53 patients. Same analysis was repeated considering variables with P<0.20. The level of significance was P<0.05. Data are presented as hazard ratios (HRs) with 95% confidence intervals (CIs). Statistical analyses were performed using SPSS version 13 (SPSS, Chicago, IL, USA) program.
Results
Methylation-dependent transcriptional silencing of DUSP2 in HNSCC cell lines
Analysis of DUSP2 expression by qRT-PCR showed that the mRNA was undetectable in HNO91 and CAL33, low in HNO41 and CAL27 and high in HEp-2 cell lines. Pyrosequencing revealed that the DUSP2 CpG island was unmethylated in HEp-2 cells and methylated in the other cell lines, with a clear correlation between methylation and DUSP2 down-regulation (Figure S1).

Methylation analysis in primary LA-HNSCC tissues
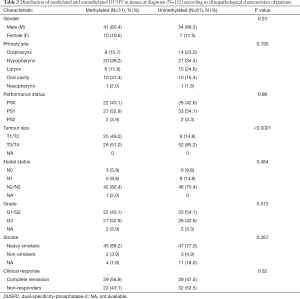
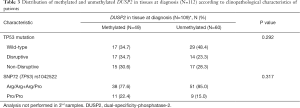
We performed pyrosequencing analysis of DUSP2 CpG island methylation in a well-annotated series of 112 cases LA-HNSCC from our clinical practice. We excluded patients with obscure cancers (N=3) and HPV16 positive oropharynx tumours (N=14). Clinico-pathological details of the cases are shown in Tables 2 and 3. Using 10% mean methylation through methylation variable sites of the amplified fragment of the CpG island as a cut-off, 51/112 (45.5%) of cases were deemed positive for DUSP2 methylation. Methylation positivity was more frequent among patients with small tumour size (T1/T2) (25/51=49.0% methylated vs. 9/61=14.8% unmethylated; P<0.0001), but there was no significant correlation between methylation status and gender, primary tumour site, PS, N, G, smoke and clinical response. Furthermore, there was no association between DUSP2 methylation and TP53 mutational status or the rs1042522 polymorphism.
Full table
Full table
DUSP2 methylation in combination with TP53 status predicts outcome in LA-HNSCC
We asked if methylation of DUSP2 is a biomarker of clinical outcome in LA-HNSCC treated with CRT, by analysis of time-dependent end points in cases positive or negative for DUSP2 methylation. There was no difference between cases positive (N=49) and negative for DUSP2 methylation (N=55) in either OS (P=0.64) nor PFS (P=0.76).
Since DUSP2 is a transcriptional target for p53, we next asked whether TP53 and DUSP2 status together might influence outcomes in the 101 patients for which both parameters were available. We identified 59 mutated patients (58.4%), with 46 missense (78%) and 13 deletions with frameshift effects (22%). In univariate analysis, no difference in OS or PFS was found among patients carrying low (N=22) vs. high (N=24) missense mutations vs. deletions (N=13), according to EAp53 analysis (29). The same result was obtained when comparing patients carrying disruptive (N=27) vs. non-disruptive (N=32) mutations, according to Poeta et al. [2007] (28). This allowed us to consider the 59 patients with TP53 mutation as a unique group (TP53-mut) in subsequent analyses. OS was longer in TP53-mut vs. TP53-wt (N=42) cases (P=0.03) (Figure 1A). This was not observed in PFS.

Combining TP53 with DUSP2 (N=101) and stratifying for TP53 status, we found that among the 54 unmet-DUSP2 cases, those carrying TP53 mutations (29/54; 53.7%) showed a longer OS (P=0.012), compared to cases with TP53-wt (25/54; 46.3%) (P=0.064) (Figure 1B). Again, no difference was found in PFS. Instead, in the 47 met-DUSP2 patients, the stratification for TP53 didn’t show any significant correlation.
DUSP2 methylation status predicts outcome in LA-HNSCC when combined with EGFR
Cases with low EGFR (N=19) had better outcome compared to patients with high-EGFR expression (N=53) although this was not significant (P=0.42). Combining EGFR with DUSP2 methylation status, we observed that cases with low EGFR and unmethylated DUSP2 (N=10) had longer OS than those with low-EGFR and met-DUSP2 (N=9) (P=0.013) (Figure 2A). On the contrary, in cases with high EGFR expression (N=53), no difference was seen in OS, although there was a trend suggesting this (Figure 2B). There was no difference in PFS in any EGFR/DUSP2 combination.

Multivariate analysis for DUSP2/EGFR/TP53 combination markers
Finally we tested the utility of the DUSP2/EGFR/TP53 triple combination in predicting clinical outcome in the 70 patients characterised for all those markers. The cumulative P value resulted significant (P=0.005). DUSP2-unmet/high-EGFR/TP53-wt makeup (N=20) was identified as the major risk factor associated with shorter OS (P=0.0007) (Figures 3,4).
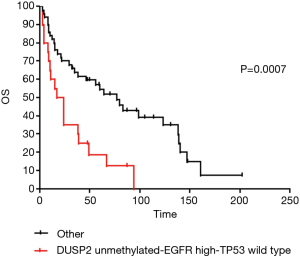

Instead, patients with DUSP2-met/high-EGFR/TP53-wt (N=4) showed the longest OS and the highest HR, although not significant, due to their small number (Figure 4A,B,C).
Discussion
In the present study we have identified DUSP2, a negative regulator of MAP kinases (6), as a novel gene subject to methylation-dependent transcriptional silencing in LA-HNSCC and we show that the quantitative level of DUSP2 methylation, when combined with EGFR expression and TP53 mutational status, has utility as a candidate biomarker of clinical outcome in patients treated with CRT. To the best of our knowledge, this is the first demonstration that CpG island methylation regulates DUSP2 expression in HN cell lines and it is methylated in clinical cases, with 45% of cases in our LA-HNSSC series positive for methylation at diagnosis. Some studies have previously reported the epigenetic inactivation of DUSP2 in human cancer cell lines (wherein methylation is associated with transcriptional silencing) but not in clinical cases (33).
In vitro, expression of DUSP2 induces apoptosis, inhibits tumour growth and abolishes hypoxia-induced drug resistance (15). These biological properties are all features of tumour suppressor genes and our demonstration of methylation-dependent transcriptional silencing of DUSP2 in LA-HNSCC affords further experimental evidence in support of this hypothesis. DUSP2 expression is a determinant of cellular sensitivity to some cytotoxic agents, including cisplatin (15) and targeted therapies such as lapatinib (34). Although DUSP2 mRNA down-regulation occurs in a number of solid tumours, increased expression levels of DUSP2 predict a worse OS in serous ovarian carcinoma (35). This apparent contradiction has recently been reported in other potential tumour-suppressor genes (36) including DUSP1 (7) and DUSP6 (37).
Low expression levels of DUSP2 correlates with reduced relapse-free survival in ERBB2-positive breast cancer patients (34), prompting us to examine DUSP2 methylation in combination with EGFR. Surprisingly, we found that LA-HNSCC cases with DUSP2 methylation (and therefore likely expressing low levels of DUSP2) and high-EGFR had increased OS compared with patients with DUSP2 unmethylated cases (although this trend did not reach significance), This effect might be associated with a favourable effect of MAPKs that are not inhibited by DUSP2 {Givant-Horwitz et al. [2004]} (35). Instead, low-EGFR patients with DUSP2-met tissues showed a shorter OS compared to patients with DUSP2-unmet tissues. Our interest in DUSP2 originated from studies showing that it is also a direct transcriptional target of wild-type p53 and may participate in the TP53-dependent DNA damage response to oxidative stress (24). Although some data suggest that TP53 might be used to predict outcome in HNSCC, its analysis has not become part of the routine evaluation in clinical settings and its role as a prognostic marker remains controversial (38).
In our cohort of LA-HNSCC, TP53 was the only biomarker, among those analysed, able to independently predict longer OS. Interestingly, we observed longer OS in cases with wild-type TP53 (58%) compared with mutant TP53. This is in apparent contradiction with outcome of CRT as suggested by others (27). Nonetheless, stratifying cases by DUSP2 methylation status, we observed that those with DUSP2 methylation and wild-type TP53 showed a numerical improved OS compared to TP53 mutant, while, in DUSP2 unmethylated cases, those with mutant TP53 showed longer OS.
Mechanistically, it might be speculated that the reduced expression of DUSP2 induces both STAT3 and MAPKs activation in tumours with intact TP53, while the consequence of normal expression of DUSP2 in mutated TP53 cases may increase cellular sensitivity to CRT (39,40).
When we analysed together the markers DUSP2/EGFR/TP53, we found that DUSP2 silencing at diagnosis was associated with more favourable clinical outcomes when combined with EGFR overexpression and wild-type TP53-wt. This highlights the relevance of intact p53 activity for the suppression of HNSCC development.
In conclusion, we show that DUSP2 methylation might have utility in predicting OS of CRT-treated LA-HNSCC patients suggesting that it may have utility as a biomarker of response to CRT therapies.
DUSP2 function in cancer supports the need for larger clinical studies to further investigate the role of this molecule.
Acknowledgements
We would like to thank Gerard Milano from Centre Antoine Lacassagne (Nice, France) for CAL27 and CAL33. This work has been partially supported by AIRC (Associazione Italiana per la Ricerca sul Cancro) Foundation (to CLN and MCM) and ARCO (Attività Ricerca Clinica Oncologica) Foundation.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study and the informed consent for biological samples collection and research proposal, obtained from patients, were approved by the Ethical Committee of S. Croce & Carle Teaching Hospital in Cuneo (approval No. 198/13).
References
- Merlano M. Alternating chemotherapy and radiotherapy in locally advanced head and neck cancer: an alternative? Oncologist 2006;11:146-51. [Crossref] [PubMed]
- Bergamaschi D, Gasco M, Hiller L, et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 2003;3:387-402. [Crossref] [PubMed]
- Vivenza D, Gasco M, Monteverde M, et al. MDM2 309 polymorphism predicts outcome in platinum-treated locally advanced head and neck cancer. Oral Oncol 2012;48:602-7. [Crossref] [PubMed]
- Vivenza D, Monteverde M, Lattanzio L, et al. Correlation of TP53 and MDM2 genotypes and clinical outcome in platinum-treated head and neck cancer patients with more than 10 years' follow-up. Int J Biol Markers 2016;31:e183-92. [Crossref] [PubMed]
- Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev 2008;27:253-61. [Crossref] [PubMed]
- Rohan PJ, Davis P, Moskaluk CA, et al. PAC-1: a mitogen-induced nuclear protein tyrosine phosphatase. Science 1993;259:1763-6. [Crossref] [PubMed]
- Patterson KI, Brummer T, O'Brien PM, et al. Dual-specificity phosphatases: critical regulators with diverse cellular targets. Biochem J 2009;418:475-89. [Crossref] [PubMed]
- Zhang Q, Muller M, Chen CH, et al. New insights into the catalytic activation of the MAPK phosphatase PAC-1 induced by its substrate MAPK ERK2 binding. J Mol Biol 2005;354:777-88. [Crossref] [PubMed]
- Wei W, Jiao Y, Postlethwaite A, et al. Dual-specificity phosphatases 2: surprising positive effect at the molecular level and a potential biomarker of diseases. Genes Immun 2013;14:1-6. [Crossref] [PubMed]
- Treinies I, Paterson HF, Hooper S, et al. Activated MEK stimulates expression of AP-1 components independently of phosphatidylinositol 3-kinase (PI3-kinase) but requires a PI3-kinase signal To stimulate DNA synthesis. Mol Cell Biol 1999;19:321-9. [Crossref] [PubMed]
- Bancroft CC, Chen Z, Dong G, et al. Coexpression of proangiogenic factors IL-8 and VEGF by human head and neck squamous cell carcinoma involves coactivation by MEK-MAPK and IKK-NF-kappaB signal pathways. Clin Cancer Res 2001;7:435-42. [PubMed]
- Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors 2006;24:21-44. [Crossref] [PubMed]
- Meloche S, Pouysségur J. The ERK1/2 mitogen-activated protein kinase pathway as a master regulator of the G1- to S-phase transition. Oncogene 2007;26:3227-39. [Crossref] [PubMed]
- Lu Z, Xu S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006;58:621-31. [Crossref] [PubMed]
- Lin SC, Chien CW, Lee JC, et al. Suppression of dual-specificity phosphatase-2 by hypoxia increases chemoresistance and malignancy in human cancer cells. J Clin Invest 2011;121:1905-16. [Crossref] [PubMed]
- Gioeli D, Mandell JW, Petroni GR, et al. Activation of mitogen-activated protein kinase associated with prostate cancer progression. Cancer Res 1999;59:279-84. [PubMed]
- Sebolt-Leopold JS, Dudley DT, Herrera R, et al. Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo. Nat Med 1999;5:810-6. [Crossref] [PubMed]
- Dhillon AS, Hagan S, Rath O, et al. MAP kinase signalling pathways in cancer. Oncogene 2007;26:3279-90. [Crossref] [PubMed]
- Kim SC, Hahn JS, Min YH, et al. Constitutive activation of extracellular signal-regulated kinase in human acute leukemias: combined role of activation of MEK, hyperexpression of extracellular signal-regulated kinase, and downregulation of a phosphatase, PAC1. Blood 1999;93:3893-9. [PubMed]
- Lin SC, Hsiao KY, Chang N, et al. Loss of dual-specificity phosphatase-2 promotes angiogenesis and metastasis via up-regulation of interleukin-8 in colon cancer. J Pathol 2017;241:638-48. [Crossref] [PubMed]
- Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol 2006;24:2666-72. [Crossref] [PubMed]
- Franovic A, Gunaratnam L, Smith K, et al. Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer. Proc Natl Acad Sci U S A 2007;104:13092-7. [Crossref] [PubMed]
- Garvalov BK, Foss F, Henze AT, et al. PHD3 regulates EGFR internalization and signalling in tumours. Nat Commun 2014;5:5577. [Crossref] [PubMed]
- Yin Y, Liu YX, Jin YJ, et al. PAC1 phosphatase is a transcription target of p53 in signalling apoptosis and growth suppression. Nature 2003;422:527-31. [Crossref] [PubMed]
- El-Deiry WS. The role of p53 in chemosensitivity and radiosensitivity. Oncogene 2003;22:7486-95. [Crossref] [PubMed]
- Tonissi F, Lattanzio L, Astesana V, et al. Reoxygenation Reverses Hypoxia-related Radioresistance in Head and Neck Cancer Cell Lines. Anticancer Res 2016;36:2211-5. [PubMed]
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35. [Crossref] [PubMed]
- Poeta ML, Manola J, Goldwasser MA, et al. TP53 mutations and survival in squamous-cell carcinoma of the head and neck. N Engl J Med 2007;357:2552-61. [Crossref] [PubMed]
- Neskey DM, Osman AA, Ow TJ, et al. Evolutionary Action Score of TP53 Identifies High-Risk Mutations Associated with Decreased Survival and Increased Distant Metastases in Head and Neck Cancer. Cancer Res 2015;75:1527-36. [Crossref] [PubMed]
- Lattanzio L, Denaro N, Vivenza D, et al. Elevated basal antibody-dependent cell-mediated cytotoxicity (ADCC) and high epidermal growth factor receptor (EGFR) expression predict favourable outcome in patients with locally advanced head and neck cancer treated with cetuximab and radiotherapy. Cancer Immunol Immunother 2017;66:573-9. [Crossref] [PubMed]
- Merlano MC, Denaro N, Vivenza D, et al. p16 cutoff in head and neck squamous cell carcinoma: correlation between tumor and patient characteristics and outcome. Int J Biol Markers 2016;31:e44-52. [Crossref] [PubMed]
- Lo Nigro C, Wang H, McHugh A, et al. Methylated tissue factor pathway inhibitor 2 (TFPI2) DNA in serum is a biomarker of metastatic melanoma. J Invest Dermatol 2013;133:1278-85. [Crossref] [PubMed]
- Haag T, Richter AM, Schneider MB, et al. The dual specificity phosphatase 2 gene is hypermethylated in human cancer and regulated by epigenetic mechanisms. BMC Cancer 2016;16:49. [Crossref] [PubMed]
- Karakashev SV, Reginato MJ. Hypoxia/HIF1α induces lapatinib resistance in ERBB2-positive breast cancer cells via regulation of DUSP2. Oncotarget 2015;6:1967-80. [Crossref] [PubMed]
- Givant-Horwitz V, Davidson B, Goderstad JM, et al. The PAC-1 dual specificity phosphatase predicts poor outcome in serous ovarian carcinoma. Gynecol Oncol 2004;93:517-23. [Crossref] [PubMed]
- Li Y, Guessous F, Kwon S, et al. PTEN has tumor-promoting properties in the setting of gain-of-function p53 mutations. Cancer Res 2008;68:1723-31. [Crossref] [PubMed]
- Messina S, Frati L, Leonetti C, et al. Dual-specificity phosphatase DUSP6 has tumor-promoting properties in human glioblastomas. Oncogene 2011;30:3813-20. [Crossref] [PubMed]
- Thomas J. Ow "TP53 as a Biomarker in Head and Neck Squamous Cell Carcinoma" (2011). UT GSBS Dissertations and Theses (Open Access). 217. Available online: http://digitalcommons.library.tmc.edu/utgsbs_dissertations/217
- Bradford CR, Zhu S, Ogawa H, et al. P53 mutation correlates with cisplatin sensitivity in head and neck squamous cell carcinoma lines. Head Neck 2003;25:654-61. [Crossref] [PubMed]
- Eriksen JG, Alsner J, Steiniche T, et al. The possible role of TP53 mutation status in the treatment of squamous cell carcinomas of the head and neck (HNSCC) with radiotherapy with different overall treatment times. Radiother Oncol 2005;76:135-42. [Crossref] [PubMed]


