Prolonged dexmedetomidine infusion in critically ill adult patients: a retrospective analysis of a large clinical database Multiparameter Intelligent Monitoring in Intensive Care III
Introduction
Dexmedetomidine is becoming more widely used as a sedative-analgesic drug in the recent years (1). It works by highly selectively exciting central α2-adrenoceptor without inhibitory effects on respiration (2). The US Food and Drug Administration (FDA) approved dexmedetomidine as a short-term analgesia and sedation medication (<24 h) in the intensive care unit (ICU) (3). The reason for its short-term use was mainly due to the risk of withdrawal side effects (e.g., rebound tachycardia and hypertension). Previous researches had revealed no significant increased mortality in critically ill patients treated with dexmedetomidine compared with lorazepam or midazolam (4-6). Some studies also confirmed that dexmedetomidine has a reduction in the length of stay in the ICU compared with other routine agents (7-9). Nowadays, long-term (≥24 h) use of dexmedetomidine in the ICU is common in clinical practice. Several studies had focused on the medication time of dexmedetomidine. Fluctuations of heart rate (HR) and blood pressure (BP) after an abrupt discontinuation of dexmedetomidine have been always observed (10-13). However, the sample size of studies mentioned above was small and there was no reporting of hospital mortality following prolonged infusion of dexmedetomidine.
Multiparameter Intelligent Monitoring in Intensive Care III (MIMIC-III) database is an open accessible critical care database comprising deidentified clinical data associated with over 40,000 patients who admitted in the ICU of the Beth Israel Deaconess Medical Center from 2001 to 2012 (14). The MIMIC-III database includes millions of records about demographics, diagnoses, vital signs, laboratory measurements, medications, survival data, etc.
In the present study, we aimed to investigate the safety of prolonged dexmedetomidine infusion in a large ICU patients cohort from the MIMIC-III database, including the effect on the hospital mortality and the withdrawal reaction.
Methods
Database
We used the MIMIC-III database (version 1.4) for the study. MIMIC-III was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA, USA) and the Massachusetts Institute of Technology (Cambridge, MA, USA). Informed consent was waived because of the nature of observation study and all deidentified protected health information. The author Gao and Zhou have got access to the database after completion of the training course “Protecting Human Research Participants” (certification number: 5999328 and 5965432).
Study population
Adult patients receiving dexmedetomidine were included in our study. The inclusion criteria were: (I) patients who were older than 18 years old, we also excluded patients who were over 89 years because MIMIC-III database has shifted their date of birth to obscure their age; (II) length of stay in the ICU was over 24 h; (III) patients with dexmedetomidine medication by continuous slow infusion (IV drip or IV pump); (IV) the infusion time of dexmedetomidine lasts for at least 1 h and no more than 100 h, because of more record and calculation errors due to long term intermittent administration (like >100 h). For patients who were admitted to the ICU more than once, we set individual ICU admission as the basic sampling unit, but not individual patients. Therefore, the same patient may be included repeatedly with different ICU admissions.
Data extraction
We performed the data extraction by using structure query language (SQL) with pgAdmin PostgreSQL tools (version 1.22.1) (15). Following health information were extracted: age, gender, weight and height at the first ICU admission, length of stay in hospital, length of stay in the ICU, hospital expire flag (in-hospital death recorded in the hospital database), BP, HR. The age was accurate to day. We calculated the body mass index (BMI) as weight in kilograms divided by the square of the height in meters. Furthermore, we calculated the Sequential Organ Failure Assessment (SOFA) scores for each patient, which represented the severity of illness and determine the extent of a person’s organ function (16). We obtained the list of anonymized patients using dexmedetomidine from the table inputevents_cv, inputevents_mv and chartevents by the corresponding ITEMIDs.
- chartevents: 1713, 2091, 2166, 2188, 2195, 2196, 2201, 2283, 2296, 2547, 8192
- inputevents_cv: 30167, 42062, 41962, 42407, 43136, 46301, 46492
- inputevents_mv: 225150
Dexmedetomidine administration time was calculated as the endpoint minus start timestamp according to continuous medication records. Besides, we also calculated accumulated doses of dexmedetomidine for all patients. We defined it as gradual reduction when the infusion rate at the endpoint is no more than half of the maximum infusion rate. Otherwise, we regarded it as an abrupt cessation. The primary outcome of present study was death in the hospital. These deaths included both death in ICU and hospital. The secondary outcome was the rebound effect of BP and HR.
Missing data handling
It is common with missing data in the MIMIC-III database. If a variable had less than 5% missing, the missing observations were omitted directly in further analysis. As for those variables with more than 5% observations missing, we explored and visualized them with Templ’s method (R Package “VIM”) (17). For randomly missing data, we used multiple imputation method (R Package “mice”) for further analysis (18).
Statistical analysis
We grouped patients by different dexmedetomidine administration time: <24 and ≥24 h. Other variables were displayed and compared between groups. We performed multivariate analysis to determine the impact of prolonged administration of dexmedetomidine on in-hospital mortality. Independent risk factors to in-hospital mortality were revealed and confounding factors were excluded.
To explore the withdrawal reaction, we extracted all records of HR and BP during 48 h after discontinuance of dexmedetomidine. Contour maps were drawn by R package “ggplot2” to measure the fluctuations of HR and BP (records during 48 h after discontinuance minus the mean records during infusion) (19). Local polynomial regression fitting were applied to build the contour maps (20).
The binary variables were compared using chi-square tests. The ordinal categorical variables and continuous variables were compared using Mann-Whitney U test. We used logistic regression model for multivariate analysis. All statistics and visualizations were performed using R version 3.3.1 software (Institute for Statistics and Mathematics, Vienna, Austria; https://www.r-project.org/). Statistical significance was set at two-sided P<0.05.
Results
We finally got 1,946 records including 1,368 distinct individuals. They were at the age from 18.23 to 89.72 and the median age was 61.36. The infusion rate was less than 2 μg/kg/h in more than 99.7% cases, and the median rate was 0.50 μg/kg/h. The total in-hospital mortality rate was 5.6%.
The baseline characteristics were briefly summarized in Table 1. There were 1,417 records with a prolonged dexmedetomidine infusion over 24 h, and 529 records less than 1 day. Patients with prolonged dexmedetomidine infusion had a longer stay in hospital and ICU (15.89 vs. 9.63 days; 10.56 vs. 3.45 days; P<0.001). We observed a higher SOFA score in prolonged dexmedetomidine infusion group (P<0.01). More importantly, prolonged dexmedetomidine infusion group had a higher risk of in-hospital death (P<0.01). We could not find difference between groups when considering other variables, including age (P=0.37), gender (P=0.72), and BMI (P=0.89).

Full table
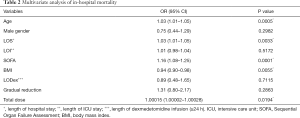
The results of multivariate analysis were summarized in Table 2. Age, BMI, length of stay in hospital, accumulated doses of dexmedetomidine and SOFA score were independent risk factors of in-hospital mortality (P<0.05). Interestingly, prolonged dexmedetomidine infusion and gradual cessation did not increase in-hospital mortality, the same as gender and length of stay in ICU (P>0.05).
Full table
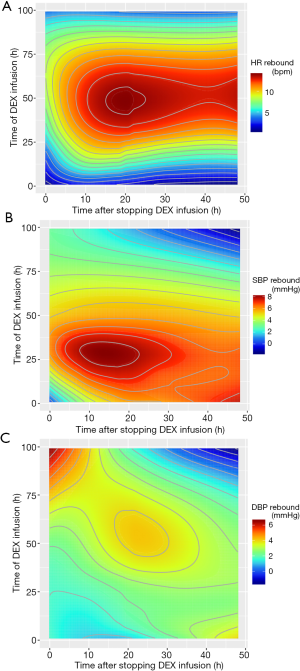
Fifty-two thousand and seven hundred fifty-five records of HR, 68,349 records of systolic BP (SBP) and 52,808 records of diastolic BP (DBP) after dexmedetomidine discontinuance were extracted. Figure 1 showed the fluctuations under distinct dexmedetomidine medication time and discontinuance time. For HR, the most violent rebound (13–14 bps) could be observed several hours after continuous infusion of dexmedetomidine for about 25 or more hours, and lasted about 20 to 35 h (Figure 1A). For SBP, the most violent rebound (about 8 mmHg) appeared several hours after continuous infusion of dexmedetomidine for about 15 to 40 h, and lasted about 30 h (Figure 1B). And for DBP, the violent rebound (about 5 mmHg) came 15 h after continuous infusion of dexmedetomidine for about 30 or more hours, and lasted about 20 h (Figure 1C).
Discussion
There is no conclusive evidence for the safety of long-term dexmedetomidine infusion. In the present study, we investigated the safety of long-term dexmedetomidine infusion, by mining the MIMIC-III database. We detected that age, in-hospital time, SOFA score and BMI were independent risk factors for in-hospital mortality. It was consistent with the mainstream views (21-23). Patients with long-term infusion and abrupt cessation were not exposed to an increased in-hospital mortality, but accumulated doses of dexmedetomidine were related with a higher risk of in-hospital mortality. Notably, higher accumulated doses were not equal to prolonged infusion. Thus, when we consider the safety of dexmedetomidine, we may need to focus on the total doses in addition to the usage time. Abrupt cessation or gradual reduction may affect the safety of medicines, but which was not observed in our study. The potential reason may be that the dosage of dexmedetomidine is relatively fixed, and the rate of 0.2–0.7 ug/kg/h is commonly used for sedative maintenance in clinical practice.
With the contour maps, we showed the rebound effect of HR and BP at the circumstance of distinct medication time and cessation time. The rebound effects were more likely to be concentrated on the prolonged medication group. In other words, our study supports that the rebound of HR and BP is more likely to occur in patients with prolonged infusion of dexmedetomidine. However, we could not observe a 20% clinically significant rebound of SBP or HR. Previous studies reported mild increase after terminating the infusion of dexmedetomidine. Carney et al. and Shehabi et al. found patients receiving dexmedetomidine for more than 24 h were more likely to have hypertension, which were similar with our study (10,12). On the contrary, Ozaki et al. supported that the increase of mean arterial BP and HR after cessation of dexmedetomidine was not associated with the increasing infusion time (13). All studies above had the limited sample size.
One strength of the present study is the data mining of a large critical care database MIMIC-III, with large sample size. The secondary analysis of “real world” data may be more generalizable compared with the strict and well-designed RCTs (24,25). In addition, there are some limitations in the present study. First, our study is a retrospective analysis, it is inevitable to bear some inherent limitations of such design. Second, critically ill patients are heterogeneous, unknown factors may still confound the results even though we have adjusted the confounding factors by multivariate analysis. Finally, we set the “in-hospital mortality” as the study outcome, which it reflected the short-term outcomes. So, the relationship of dexmedetomidine and long-term outcomes is still unclear.
Conclusions
In conclusion, the study shows that prolonged dexmedetomidine infusion is not related to an increased in-hospital mortality, but it is associated with the rebound effects of heart rate and blood pressure. The findings require further prospective studies to confirm.
Acknowledgements
The authors appreciate the efforts of the Medical Information Mart for Intensive Care III Database. Thanks to all code contributors in GitHub across the world as well as researchers at the MIT Laboratory for Computational Physiology and collaborating research groups.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Short J. Use of dexmedetomidine for primary sedation in a general intensive care unit. Crit Care Nurse 2010;30:29-38. [Crossref] [PubMed]
- Fuchs B, Bellamy C. Sedative-analgesic medications in critically ill adults: Properties, dosage regimens, and adverse effects. Available online: https://www.uptodate.com/contents/sedative-analgesic-medications-in-critically-ill-adults-selection-initiation-maintenance-and-withdrawal
- Gertler R, Brown HC, Mitchell DH, et al. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13-21. [PubMed]
- Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA 2007;298:2644-53. [Crossref] [PubMed]
- Akada S, Takeda S, Yoshida Y, et al. The efficacy of dexmedetomidine in patients with noninvasive ventilation: a preliminary study. Anesth Analg 2008;107:167-70. [Crossref] [PubMed]
- Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301:489-99. [Crossref] [PubMed]
- Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med 2010;36:926-39. [Crossref] [PubMed]
- Pasin L, Greco T, Feltracco P, et al. Dexmedetomidine as a sedative agent in critically ill patients: a meta-analysis of randomized controlled trials. PLoS One 2013;8. [Crossref] [PubMed]
- Chen K, Lu Z, Xin YC, et al. Alpha-2 agonists for long-term sedation during mechanical ventilation in critically ill patients. Cochrane Database Syst Rev 2015;1. [PubMed]
- Shehabi Y, Ruettimann U, Adamson H, et al. Dexmedetomidine infusion for more than 24 hours in critically ill patients: sedative and cardiovascular effects. Intensive Care Med 2004;30:2188-96. [Crossref] [PubMed]
- Guinter JR, Kristeller JL. Prolonged infusions of dexmedetomidine in critically ill patients. Am J Health Syst Pharm 2010;67:1246-53. [Crossref] [PubMed]
- Carney L, Kendrick J, Carr R. Safety and Effectiveness of Dexmedetomidine in the Pediatric Intensive Care Unit (SAD–PICU). Can J Hosp Pharm 2013;66:21-7. [Crossref] [PubMed]
- Ozaki M, Takeda J, Tanaka K, et al. Safety and efficacy of dexmedetomidine for long-term sedation in critically ill patients. J Anesth 2014;28:38-50. [Crossref] [PubMed]
- Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data 2016;3. [Crossref] [PubMed]
- Zhang Z. Accessing critical care big data: a step by step approach. J Thorac Dis 2015;7:238-42. [PubMed]
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996;22:707-10. [Crossref] [PubMed]
- Templ M, Alfons A, Filzmoser P. Exploring incomplete data using visualization techniques. Adv Data Anal Classif 2012;6:29-47. [Crossref]
- van Buuren S, Groothuis-Oudshoorn CG. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011;45:67.
- Wickham H. ggplot2. WIREs Comp Stat 2011;3:180-5. [Crossref]
- Fan J, Gasser T, Gijbels I, et al. Local Polynomial Regression: Optimal Kernels and Asymptotic Minimax Efficiency. Ann I Stat Math 1997;1:79-99. [Crossref]
- Goulenok C, Monchi M, Chiche JD, et al. Influence of overweight on ICU mortality: a prospective study. Chest 2004;125:1441-5. [Crossref] [PubMed]
- Hein OV, Birnbaum J, Wernecke K, et al. Prolonged intensive care unit stay in cardiac surgery: risk factors and long-term-survival. Ann Thorac Surg 2006;81:880-5. [Crossref] [PubMed]
- Minne L, Abu-Hanna A, de Jonge E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care 2008;12:R161. [Crossref] [PubMed]
- Wang SD. Opportunities and challenges of clinical research in the big-data era: from RCT to BCT. J Thorac Dis 2013;5:721-3. [PubMed]
- Zhang Z, Chen K, Ni H. Calcium supplementation improves clinical outcome in intensive care unit patients: a propensity score matched analysis of a large clinical database MIMIC-II. Springerplus 2015;4:594. [Crossref] [PubMed]


