
Efficacy and safety of Shen-Yuan-Dan capsules for peri-procedural myocardial injury following percutaneous coronary intervention: study protocol for a randomized, double-blind, placebo-controlled trial
Introduction
With the widespread use of percutaneous coronary intervention (PCI) in the treatment of coronary heart disease, peri-procedure myocardial injury (PMI) has attracted more and more attention. According to the Third Universal Definition of Myocardial Infarction released by ESC/ACCF/AHA/WHF in 2012, PMI was defined as an increase in myocardial biomarkers after PCI (1). Studies have shown that the incidence of PMI was 15.8-30%, and its severity is closely associated with the incidence of major adverse cardiovascular events (MACEs) (2-4). Besides conventional anti-platelet, anticoagulant, and other treatments, the use of loading doses of statins before PCI is currently considered to be an important therapeutic measure for decreasing PMI (5-8). However, there is still a controversy concerning whether this method is effective in Asian populations (9). In the China percutaneous coronary intervention guidelines [2016], statin loading before PCI is not recommended (10). Therefore, whether treatment drugs for myocardial protection during the peri-procedural phase of PCI could be discovered from traditional medicine, has become a focus for research (particularly in China) (11-13).
Shen-Yuan-Dan (SYD) is a herbal preparation that comprises Astragalus membranaceus, Salvia miltiorrhiza, Codonopsispilosula, Scrophularia ningpoensis, Corydalis ambigua, Hirudo nipponica, Eupolyphaga sinensis, and Pheretima (Table 1). Studies have shown that SYD cannot only improve the clinical symptoms of patients with unstable angina, but can also elicit a myocardial protection effect, through endothelial protection, anti-oxidative stress, and other mechanisms (14-17).
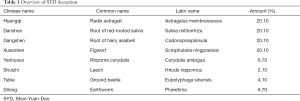
Full table
Previously, we performed a basic study using Chinese miniature pigs to establish a PMI model by carrying out a balloon dilation, strain, and stent implantation in a coronary stenosis, in the pigs. The study showed that SYD could reduce PMI, and this effect seemed to be more significant in the high-dose group when compared with the low-dose group (18). Based on the results of the above study, we recently carried out a small-sample clinical trial to evaluate whether SYD could decrease PMI. The preliminary experiment results showed that SYD has potential for myocardial protection during the PCI peri-procedural phase. However, further evaluation on the basis of a reasonable sample size is still required. Therefore, this study will aim to combine the research foundation of the preliminary experiments to carry out a rigorous, randomized, controlled trial for further evaluation of the efficacy and safety of SYD in decreasing PMI.
Methods and design
Experimental design
This is a randomized, double-blind, placebo-controlled clinical trial (Figure 1). This study will be carried out at the Beijing Hospital of Traditional Chinese Medicine, affiliated with the Capital Medical University. A total of 284 patients with unstable angina and, who fulfill the inclusion criteria, will be randomized into test and control groups. The two groups will be either given SYD or a placebo (three times each day, four capsules each time) 3 days before PCI on the basis of conventional treatment. Twelve hours before PCI, 4 additional capsules will be given, and drug treatment will be maintained for 1 month after surgery. Dynamic changes in the myocardial enzyme in four time-points (before PCI, and 4, 24, 48 hours after PCI) in both groups of patients will be observed. The follow-up period will be 1 month. For evaluation of the efficacy and safety of SYD in decreasing PMI, the primary therapeutic outcome markers will be determined by the incidence of PMI in both groups as indicated by an increase of more than twice the normal upper limit in troponin T (TnT) within 48 hours after PCI. The secondary observation markers will be the MACEs status evaluations at day 30 after PCI (all-cause mortality, non-fatal myocardial infarction, repeated revascularization of target blood vessel) and Seattle Angina Questionnaire scores. GRACE scores will be used for risk stratification, and the intervention efficacy of SYD on PMI patients with different risks will be retrospectively evaluated. Safety outcome markers include severe kidney impairment and severe clinical discomfort. This study strictly will conform to the Declaration of Helsinki and the guiding principles for clinical trials. The ethical consent form will be approved by the ethics committee of the Beijing Hospital of Traditional Chinese Medicine affiliated to the Capital Medical University.
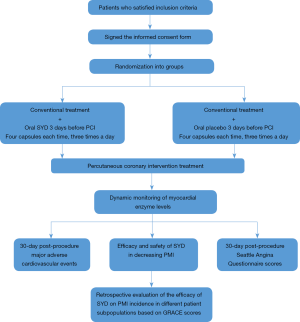
Participation and recruitment
Inclusion criteria
The inclusion criteria of this study include the following patient parameters: (I) age 18–85 years; (II) fulfilling the diagnosis criteria for unstable angina; (III) agreeing to undergo an elective coronary angiography and coronary intervention treatment; (IV) voluntarily signing of the informed consent form.
Exclusion criteria
Exclusion criteria include: patients with acute myocardial infarction (ST-segment elevation or non ST-segment elevation), or typical stable effort angina; patients warranting emergency coronary angiography; patients with other factors influencing measurement of cTnI levels, (such as myocarditis or cardiomyopathy) left ventricular ejection fraction <30%; patients with serious liver incompetence including aspartate aminotransferase (AST)/alanine aminotransferase (ALT) level > two times upper limit of normal (ULN); patients with renal failure including creatinine >3 mg/dL; patients with any known malignant tumor; female patients who are pregnant, lactating, or planning a pregnancy during the course of the study; patients with a history of intolerance or hypersensitivity to SYD, or previous or current treatment with SYD or another traditional Chinese medicine (TCM) prescription; patients using medications for which co-administration with clopidogrel, aspirin, nitrates, or statins are contraindicated; and patients with participation in any other studies involving investigational or marketed products within 1 month prior to entry in the study.
Withdrawal criteria
Patients who are found not to fulfill the diagnostic criteria for unstable angina after inclusion or who are mistakenly included due to coronary intervention imaging criteria will be withdrawn from this study.
Dropout criteria
Patients who are unable to be followed-up with during the follow-up period will be considered dropped out criteria out of the study.
Termination criteria
The termination criteria will include: (I) severe liver and kidney impairment after inclusion (ALT or AST more than twice the upper limit of normal, Cr >3 mg/dL or 265.2 μmol/L); (II) confirmed study drug-induced severe clinical discomfort (such as severe nausea and vomiting, severe bleeding, etc.); (III) severe drug allergy.
The inclusion, exclusion, termination, or withdrawal of patient participation require the simultaneous decision of at least two doctors with intermediate job titles.
Randomization and grouping
Patients who fulfill the criteria will be randomized into SYD and placebo groups. Randomized numbers will be generated by independent statisticians using SPSS 15.0 (PN: 32119001, SN: 5045602). Then, the randomized numbers will be ranked in order, and patients will be divided into two groups according to the corresponding odd/even numbers of the randomized numbers. The randomized numbers, rank sequence, and grouping, will be all stored in opaque envelopes that are kept by the designer and statisticians during the entire study process. All envelopes will be given a running serial number, and each include patient will receive an envelope. The physician will allocate the patient to the SYD or the placebo group according to the contents in the envelope.
Blinding method
Double-blinding of the physicians and included patients will be used in this study. In order to implement blinding, SYP and the placebo will be placed in a capsule with similar packaging, size, color, and taste. During the study, if patients experience severe adverse events, the physician will be expected to execute an emergency unblinding, and perform relevant treatment according to the situation. After the study concludes, the statisticians will carry out the first unblinding, and conduct statistical analysis based on data from the two groups. After data analysis, the designer will carry out the second unblinding to confirm which group is the SYD or placebo group. Staff in charge of collection of the outcome data and outcome evaluation, will all be third-party staff who did not participate in the design or patient recruitment of this study.
Intervention method
After grouping, patients will undergo basic drug treatment, according to the 2016 diagnosis and treatment guidelines for the non-ST segment elevation acute coronary syndrome which was released by the Chinese Medical Association (19). These drugs include anti-platelets (aspirin, clopidogrel), statins (routine doses), beta-blockers, nitrates, inhibitors of the renin-angiotensin-aldosterone system, etc. Three days before intervention treatment, patients will be given SYD or a placebo together with warm water 0.5 hours after Western medicine is given. The medication administration method will be the same for both capsules: 4 capsules each time, three times a day; with an additional 4 capsules 12 hours before PCI, and routine dosage for 1 month after PCI. SYD and the placebo are manufactured by Beijing Xinglin Pharmaceutical Industry Co. Ltd. The storage, transportation, management, recovery, and the destruction of the SYD medicine and the placebo capsules will be all handled by staff from the pharmacology department of the hospital.
Outcome evaluation
Primary outcome
Peri-procedural myocardial injury (PMI)
Using the Third Universal Definition of a Myocardial Infarction released by ESC/ACCF/AHA/WHF in 2012 (1), an increase in TnT more than two times the upper limit of normal within 48 hours after PCI, will be used as a diagnostic marker for PMI. Testing for the marker will be carried out by technicians in the department, using the Roche myocardial enzyme analyzer (Cobas H232 Cardiac System) and the upper limit of normal for TnT is 50 ng/L. Markers will be tested at the following points: before PCI, 4, 24, and 48 hours after surgery. PMI will be defined as TnT elevations greater than 100 ng/L within 48 hours after PCI. If TnT elevations within 48 hours after PCI are greater than 250 ng/L (5 times the upper limit of normal) and are accompanied by cardiac ischemia symptoms, or evidence from coronary angiography and other imaging, this will be defined as a peri-procedural myocardial infarction.
Secondary outcomes
30-day post-procedure MACEs
Outpatient referral or telephone follow-up will be used by 3rd party observation staff to record the 30-day MACE status of patients. The MACE events used in this study will include, all-cause mortality, non-fatal myocardial infarction, and repeated revascularization of target blood vessel.
30-day post-procedure Seattle Angina Questionnaire scores
Outpatient referral or telephone follow-up will be used by 3rd party observation staff to evaluate the angina scores of the patients according to the Seattle Angina Questionnaire.
Subpopulation analysis according to risk stratification
Patients that are finally included in the study will be stratified according to their coronary heart disease risk (GRACE scores), with the low risk group being 1–108 points, the moderate risk group being 109–140 points, and the high-risk group being 141–372 points. A retrospective analysis of the intervention efficacy of SYD on the PMI in different patient subpopulations will be carried out.
Safety evaluation
The serum biochemical tests of the patients and the appearance of discomfort after drug administration in the patients will be used to evaluate the safety of the study drug. The safety problems that occur in the patients will be recorded in a timely manner. If the patient experiences any item that fulfills the termination criteria, emergency unblinding of that patient will be carried out, and the study will be terminated. The physician will treat the patient according to the actual situation.
Sample size
This study will use sample size estimation formula according to the optimal design of the technical data, and the target event is the PMI incidence, thus the following formula will be used for estimation:
According to the results of the preliminary experiments, the incidence of PMI in the SYD and placebo groups are estimated to be 8% and 20%, respectively. Using α=0.025 (one-tailed), β=0.2 in the formula, each group will require around 129 patients. In addition, it will be estimated that ≤10% of patients will be withdrawn or lost to follow up. Therefore, a total of 284 patients will be needed.
Statistical analysis
Continuous data is expressed as mean ± standard deviations (SD). Categorical variables are expressed as frequencies (%). Continuous variables between groups will be compared using the t-test for normally distributed values. To compare normally distributed continuous variables with homogeneity of variance between groups, completely randomized design, one-way analysis of variance (ANOVA) will be used. The SNK-q test will be used for pairwise comparisons between groups. To compare non-normally distributed continuous variables between groups, the rank-sum test will be used. Comparisons between groups will be performed using the chi-square test (or Fisher’s exact test when the expected frequency is <5). Post hoc analysis will be carried out using LOGISTIC and COX regression analysis. All calculations will be performed using SPSS 15.0. The threshold for significance will be set to P<0.05 (2-tailed).
Discussion
According to our understanding, this is the first randomized, double-blind, placebo-controlled clinical trial protocol evaluating TCM preparations in reducing PMI. Currently, it is believed that tiny lipid particles produced during intervention obstructing the distal coronary blood vessel is one of the important causes of PMI occurrence. In TCM, this is believed to be a “blood stasis,” and treatment should be based on “clearing blood stasis” as its core principle. SYD is a preparation that has “qi boosting and stasis clearance” properties. Therefore, we believed that SYD could have a potential myocardial protective effect on the peri-procedural phase of PCI. Previously, our basic research study has suggested that SYD could reduce PMI and the effects were more significant in the high dose group when compared with the low dose group (18). Subsequently, we designed a small sample, clinical trial and enrolled a total of 76 unstable angina patients. These patients were randomized into a SYD group (n=26) and a placebo group (n=40) and were given SYD or a placebo 3 days before PCI, in addition to conventional treatment. However, no drug loading was given 12 hours before PCI. Results showed that the incidence of PMI in the SYD group were lower than that of the placebo group, but the difference was not statistically significant (8.3% vs. 20.0%, P=0.149). We analyzed the results, and considered that this may be related to sample size limitations. On the other hand, this may also be due to the absence of the drug loading before PCI. Referring to the results of previous studies, this study protocol is intended to compensate for the design deficiencies in the previous study.
In addition, besides evaluating the efficacy of SYD in decreasing PMI and safety, we also plan to observe the effects of SYD on the 30-day post-procedural MACE event and Seattle Angina Questionnaire score in patients and retrospectively evaluate the intervention efficacy of SYD on PMI in patients in different risk strata. This study will further evaluate the potential clinical benefits of SYD towards patients undergoing PCI treatment. Of course, the fact that this is a single-center study will be a limitation of this study design. However, this study will provide a new approach in decreasing PMI and the results of the study are worth anticipating.
Acknowledgements
Funding: This study has been supported by Grants from the National Natural Science Foundation of China (No. 81273741), Beijing Municipal Administration of Hospitals Incubating Program (No. PZ2017007), and the Natural Science Foundation of Beijing Municipality (No. 7142077). The authors are grateful for the financial support of these institutions.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This trial protocol has been approved by the Ethics Committee of Beijing Hospital of Traditional Chinese Medicine, Capital Medical University (No. 2017SB-32). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation 2012;126:2020-35. [Crossref]
- Herrmann J. Peri-procedural myocardial injury: 2005 update. Eur Heart J 2005;26:2493-519. [Crossref]
- Feldman DN, Kim L, Rene AG, et al. Prognostic value of cardiac troponin-I or troponin-T elevation following nonemergent percutaneous coronary intervention: a meta-analysis. Catheter Cardiovasc Interv 2011;77:1020-30. [Crossref]
- Babu GG, Walker JM, Yellon DM, et al. Peri-procedural myocardial injury during percutaneous coronary intervention: an important target for cardioprotection. Eur Heart J 2011;32:23-31. [Crossref]
- Di Sciascio G, Patti G, Pasceri V, et al. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol 2009;54:558-65. [Crossref]
- Pasceri V, Patti G, Nusca A, et al. Randomized trial of atorvastatin for reduction of myocardial damage during coronary intervention: results from the ARMYDA (Atorvastatin for Reduction of MYocardial Damage during Angioplasty) study. Circulation 2004;110:674-8. [Crossref]
- Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol 2007;49:1272-8. [Crossref]
- Briguori C, Visconti G, Focaccio A, et al. Novel approaches for preventing or limiting events (Naples) II trial: impact of a single high loading dose of atorvastatin on periprocedural myocardial infarction. J Am Coll Cardiol 2009;54:2157-63. [Crossref]
- Jang Y, Zhu J, Ge J, et al. Preloading with atorvastatin before percutaneous coronary intervention in statin-naïve Asian patients with non-ST elevation acute coronary syndromes: A randomized study. J Cardiol 2014;63:335-43. [Crossref]
- Section of Interventional Cardiology of Chinese Society of Cardiology of Chinese Medical Association, Specialty Committee on Prevention and Treatment of Thrombosis of Chinese College of Cardiovascular Physicians, Editorial Board of Chinese Journal of Cardiology. Chinese guideline for percutaneous coronary intervention (2016). Zhonghua Xin Xue Guan Bing Za Zhi 2016;44:382-400.
- Chu FY, Liu HX. Current status and prospects of TCM intervention research in posterior coronary artery intervention. Shi Zhen Gui Yi Guo Yao 2014;25:699-701.
- Liu HX, Wu YJ, Wang X, et al. Consensus of Traditional Chinese Medicine Specialists on Percutaneous Coronary Intervention (PCI) Perioperative Myocardial Injury. Zhongguo Zhong Xi Yi Jie He Za Zhi 2017;37:389-93.
- HX Liu. Brilliant Future for Integrative Medicine in Interventional Cardiology. Chinese Journal of Integrative Medicine on Cardio-Cerebrovascular Disease 2015:561-62.
- Liu H, Shang J, Chu F, et al. Protective Effects of Shen-Yuan-Dan, a Traditional Chinese Medicine, against Myocardial Ischemia/Reperfusion Injury In Vivo and In Vitro. Evid Based Complement Alternat Med 2013;2013:956397. [Crossref]
- Shang JJ, Li AY, Yang HZ, et al. Effect of Shenyuandan pharmacology preconditioning on rat's ischemia-reperfusion myocardial infarction size, protein kinase C and heat shock protein 70. China Journal of Traditional Chinese Medicine and Pharmacy 2011;26:730-3.
- Li AY, Wu B, Liu HX, et al. Evaluation and pharmacokinetic research of salvianic acid A in shen yuan yi qi huo xue capsules. J Beijing U Trad Med 2015;34:180-2.
- Liu HX, Jin M, Wang ZS, et al. Clinical observation on 113 cases of unstable angina (stasis syndrome) treatment with "Shen Yuan Dan" decoction. J Tradit Chin Med 1999.219-21.
- Xing WL, Tang Y, Liu HX, et al. The Effect of Shen Yuan Dan on Oxidative Stress Reaction of Chinese Miniature Pig PMI Model. World Chinese Medicine 2016;11:402-6.
- Chinese Society of Cardiology. editorial board of Chinese Journal of Cardiology. Guidelines for diagnosis and treatment of non-ST elevation acute coronary syndrome (2016). Chinese Journal of Cardiology 2017;45:359-76.


