Dead space in acute respiratory distress syndrome
Introduction
Physiological gas exchange occurs only in presence of ventilation and perfusion (V/Q) homogeneity; nevertheless an easy and accurate indicator that can measure V/Q alterations is far from being available. Dead space (Vd) is the portion of each tidal volume that does not take part in gas exchange and includes: anatomical dead space (Vdaw), that is the part of airways that do not contribute to gas exchange (nose, pharynx, conduction airways and ventilator equipment if mechanical ventilation is present) and alveolar dead space (Vdalv) or alveoli which are well-ventilated but poorly perfused (1,2).
In the continuous search for an index of the efficiency of the gas exchange in critical care patients, dead space is the only parameter that reflects the alterations in V/Q ratios and any type of V/Q mismatch affects it. Nevertheless, monitoring dead space at the bedside in this kind of patients is infrequent, especially because the capnograms are influenced by many factors related both to the patient and to the ventilator, but also to the monitoring system used, which inevitably complicate their interpretation. Moreover, over the years different methods of calculation have been proposed (3,4).
Calculation of dead space
When Bohr in 1891 calculated dead space fraction of expired tidal volume for the first time as Vd/Vt = (PACO2 − PeCO2)/PACO2, where Vt was total exhaled volume, PACO2 the amount of carbon dioxide at the alveolar level and PeCO2 the partial pressure of mean expired carbon dioxide, it was immediately evident that, if in an ideal lung arterial PCO2 (PaCO2) would be the equivalent of PACO2, this perfect condition was unachievable in clinical practice, where PACO2 was always less than PaCO2.
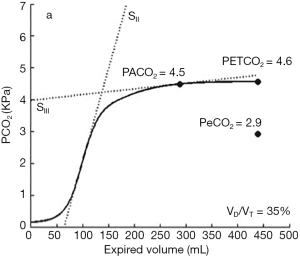
For this reason and for the difficulties with measurement of PACO2, Enghoff in 1938 used PaCO2 instead of PACO2 and adapted the Bohr’s equation as Vd/Vtphys = (PaCO2 − PECO2)/PaCO2, where PECO2 was obtained using volumetric capnography. With this technique volume and CO2 are simultaneously measured and the latter is plotted against expired volume. The resulting volumetric capnogram is composed by three phases. Phase I represents gas from the conductive airways, where CO2 is almost zero; In phase II CO2 comes from the first alveoli close to the main airways, whereas phase III is composed from pure alveolar gas. The mid-portion of the latter phase represents the most accurate estimation of mixed expired partial pressure of CO2 (PECO2) (5) (Figure 1).
Today, volumetric capnography is widely used in clinical practice and dead space variations may be a useful clinical predictor and prognostic factor.
Dead space in acute respiratory distress syndrome (ARDS)
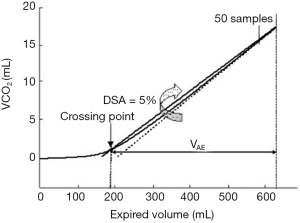
In early ARDS patients, Nuckton and colleagues demonstrated that an elevated Vd/Vtphys, measured in the first day, was a strong independent predictor of mortality. Injury of pulmonary capillaries by thrombotic and inflammatory factors, obstruction of pulmonary blood flow in pulmonary circulation and lung areas with high V/Q ratio, due to impaired CO2 excretion, were the primary determinants of an a high Vd/Vt (7). Similarly, Raurich and colleagues reported a higher risk of death in ARDS patients with increased Vd/Vtphys (8). Lucangelo and colleagues tested different dead space indices in ARDS and acute lung injury (ALI) patients and the main findings were that a computerized physiologically based index, the fraction between alveolar gas ejection volume and tidal volume (Vae/Vt), was strongly related to outcome. Briefly, the Vae was then measured from the CO2 elimination versus volume curve [VCO2(V)] curve as follows: firstly, the slope of the last 50 points of every cycle was obtained by linear regression analysis, representing an ideal lung behaviour. Vae/Vt was measured as the volume between the intersection among the VCO2(V) curve and a straight line, having a maximal value at end-expiration and a slope equal to 0.95 times the ideal line and the end of expiration (Figure 2). The need of a different approach to volumetric capnography was due to the dependence of the alveolar slope indices from the visual criterion used to define phase III (9).
Pulmonary hypertension is common in the early phase of ARDS; modifications in microvasculature are the main determinants systolic pulmonary arterial pressure (PA) elevation. In 42 patients with ALI and lung protective ventilated, Cepkova and colleagues found that not PA elevation, but Vd/Vt increase in early ALI was associated with a worse outcome (10).
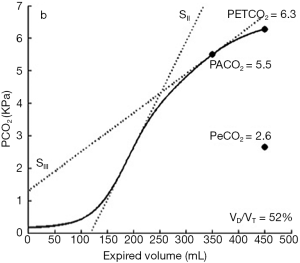
Recently, in 30 intensive care patients, volumetric capnography was used by Doorduin and colleagues to measure PACO2 and PeCO2, but the latter was obtained also using a Douglas bag, and indirect calorimetry; the results remarked the importance of a correct interpretation of dead space measures, in particular when different techniques are used. Indeed, by replacing PACO2 with PaCO2, ARDS patients showed greater variations in volumetric capnography compared to patients with normal lung function (Figure 3) (6).
Dead space and lung recruitment
The main purpose of lung recruitment is to reverse atelectasis and positive end expiratory pressure (PEEP) application allows to keep the alveoli open.
As early as 1987, Blanch and colleagues used PacO2-PetCO2 gradient to select the best PEEP level: in 13 ALI patients, the presence of a low inflection point identified potentially recruitable lungs; the latter showed a reduction in dead space after the application of PEEP (11). In six saline lavaged pigs, Tusman and colleagues compared dead space variables on a breath-by-breath basis with continuous arterial oxygenation and chest computed tomography (CT) scans and found that dead space variations were related to lung derecruitment and to establish the best PEEP after recruitment manoeuvres (RMs) (12). The same results were obtained using Vd\Vt by Fengmei and colleagues, that ventilated twenty-three ARDS patients in low Vt volume-controlled mode. In this study two important remarks arise: first, alveolar overdistension and reduction in cardiac output due to high PEEP levels could be responsible for the increase in dead space; secondly, lowest Vd\Vt corresponded to a higher PEEP value than that equivalent to the maximum static compliance (13). The latter confirmed the findings of Blanch and colleagues, which in 1999 demonstrate that the severity of the disease affects volumetric capnography and the mechanical properties of the respiratory system; increasing PEEP improved Crs in normal subjects, but did not affect volumetric capnographic indices (14). On the contrary, Beydon and colleagues (15) demonstrated in patients with various degrees of ALI that Vdalv was large and does not vary systematically with different PEEP levels; when individual measurements were done, they showed a diverse response to PEEP. In fact, high PEEP reduced pulmonary shunt and dead space, but the latter increased at the same PEEP level in different ALI patients due to alveolar overdistension and vessels compression. Both Enghoff modification of Bohr’s equation and respiratory mechanics were not able to optimize PEEP level. In sixty-eight patients with ALI or ARDS, Gattinoni and colleagues performed chest CT scans during breath-holding sessions at different PEEP levels; the portion of lung tissue in which aeration was restored depended on PEEP level. Moreover, in the group with a higher percentage of potentially recruitable lung, the respiratory-system compliance was lower, whereas the PaCO2 and the percentage of dead space were higher; the authors concluded that, in ARDS patients, the percentage of potentially recruitable lung was extremely variable and strongly associated with the response to PEEP. Best static compliance (Cst) related PEEP was characterized from an increase in dead space, that may be due overdistension; the latter occurred above highest Cst values, when improvement in compliance and oxygenation occurs below. On the other hand, Vd guided PEEP improved compliance and oxygenation with less Vd/Vt and inspiratory plateau pressure (16).
Dead space and ventilation pattern
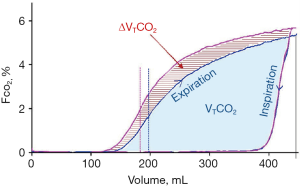
Lung-protective ventilation represents a cornerstone in ALI and ARDS patient management, but this has been associated with a reduction in alveolar ventilation and a consequent retention of carbon dioxide. Aström and colleagues tested different patterns of Vt delivery and their effect on CO2 elimination in healthy and ALI pigs, and the main finding was that a long inspiratory pause reduced Vd, and consequently PaCO2, prolonging the mean distribution time, that is the mean time during which consecutive fractions of inspired tidal volume remain in the respiratory zone of the lung (17). The same results were found in 2012 by Aboab and colleagues, which studied the effects of breathing pattern on the time available for distribution and diffusion of inspired tidal gas within resident alveolar gas in eight ARDS patients. They showed that was possible to enhance CO2 elimination by about 15% just increasing inspiratory pause duration, maintaining a constant and protective Vt; further improvement was possible by increasing inspiratory flow. The additional volume of CO2 eliminated was caused partly by a lower-airway dead space and partly by a higher level of the alveolar plateau (Figure 4) (18).
Effects on gas exchange and mechanics from prolonging end-inspiratory pause were investigated in 13 ARDS patients by Aguirre-Bermeo and colleagues in 2016. A longer end-inspiratory pause was associated at a decrease in Vt but without changes in PaCO2 levels. Moreover, Crs increased and a significant decrease in Pplat and driving pressure was observed; the authors concluded that end-inspiratory pause prolongation avoided overdistension and dynamic strain to the lung (19).
Conclusions
Knowing the pathophysiology of patients admitted to intensive care has become essential; dead space measurement is a reliable method that provides important clinical and prognostic information, in particular in ALI and ARDS patients. Volumetric capnography is today the most reliable method for measuring dead space in real time and its daily use will only improve the management of critical patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lucangelo U, Blanch L. Dead space. Intensive Care Med 2004;30:576-9. [Crossref] [PubMed]
- Suarez-Sipmann F, Bohm SH, Tusman G. Volumetric capnography: the time has come. Curr Opin Crit Care 2014;20:333-9. [Crossref] [PubMed]
- Kallet RH, Zhuo H, Liu KD, et al. The association between physiologic dead-space fraction and mortality in subjects with ARDS enrolled in a prospective multicenter clinical trial. Respir Care 2014;59:1611-8. [Crossref] [PubMed]
- Blanch L, López-Aguilar L, Lucangelo U. Dead space in acute respiratory distress syndrome: more than a feeling! Critical Care 2016;20:214. [Crossref] [PubMed]
- Fletcher R, Jonson B, Cumming G, et al. The concept of dead space with special reference to the single breath test for carbon dioxide. Br J Anaesth 1981;53:77-88. [Crossref] [PubMed]
- Doorduin J, Nollet J, Vugts M, et al. Assessment of dead-space ventilation in patients with acute respiratory distress syndrome: a prospective observational study. Critical Care 2016;20:121. [Crossref] [PubMed]
- Nuckton TJ, Alonso JA, Kallet RH, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med 2002;346:1281-6. [Crossref] [PubMed]
- Raurich JM, Vilar M, Colomar A, et al. Prognostic value of the pulmonary dead-space fraction during the early and intermediate phases of acute respiratory distress syndrome. Respir Care 2010;55:282-7. [PubMed]
- Lucangelo U, Bernabe F, Vatua S, et al. Prognostic value of different dead space indices in mechanically ventilated patients with acute lung injury and ARDS. Chest 2008;133:62-71. [Crossref] [PubMed]
- Cepkova M, Kapur V, Ren X, et al. Pulmonary dead space fraction and pulmonary artery systolic pressure as early predictors of clinical outcome in acute lung injury. Chest 2007;132:836-42. [Crossref] [PubMed]
- Blanch L, Fernández R, Benito S, et al. Effect of PEEP on the arterial minus end-tidal carbon dioxide gradient. Chest 1987;92:451-4. [Crossref] [PubMed]
- Tusman G, Suarez-Sipmann F, Bohm SH, et al. Monitoring dead space during recruitment and PEEP titration in an experimental model. Intensive Care Med 2006;32:1863-71. [Crossref] [PubMed]
- Fengmei G, Jin C, Songqiao L, et al. Dead space fraction changes during PEEP titration following lung recruitment in patients with ARDS. Respir Care 2012;57:1578-85. [Crossref] [PubMed]
- Blanch L, Lucangelo U, Lopez-Aguilar J, et al. Volumetric capnography in patients with acute lung injury: effects of positive end-expiratory pressure. Eur Respir J 1999;13:1048-54. [Crossref] [PubMed]
- Beydon L, Uttman L, Rawal R, et al. Effects of positive end-expiratory pressure on dead space and its partitions in acute lung injury. Intensive Care Med 2002;28:1239-45. [Crossref] [PubMed]
- Gattinoni L, Vagginelli F, Carlesso E, et al. Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med 2003;31:2727-33. [Crossref] [PubMed]
- Aström E, Uttman L, Niklason L, et al. Pattern of inspiratory gas delivery affects CO2 elimination in health and after acute lung injury. Intensive Care Med 2008;34:377-84. [Crossref] [PubMed]
- Aboab J, Niklason L, Uttman L, et al. Dead space and CO2 elimination related to pattern of inspiratory gas delivery in ARDS patients. Crit Care 2012;16:R39. [Crossref] [PubMed]
- Aguirre-Bermeo H, Morán I, Bottiroli M, et al. End-inspiratory pause prolongation in acute respiratory distress syndrome patients: effects on gas exchange and mechanics. Ann Intensive Care 2016;6:81. [Crossref] [PubMed]