
“The multimodal approach for ventilator-associated pneumonia prevention”—requirements for nationwide implementation
Introduction
Ventilator-associated pneumonia (VAP) is one of the main types of infection in intensive care units (ICU) (1,2). Its occurrence has been linked to increased length of ICU and hospital stay, duration of mechanical ventilation, as well as higher crude and/or attributable mortality (3). VAP rate is a quality and safety indicator of ICU care (4). The great amount of research done aiming at prevention has focused mainly on pathophysiological risk factors contributing to the development of VAP. Many studies have been published addressing specific individual VAP prevention measures, which may be classified as “functional”, “mechanical” or “pharmacological” (Table 1). At the same time, different scientific societies have analyzed, classified and recommended these measures based on their level of evidence and feasibility (5,6). Awareness and compliance of these preventive interventions are highly variable, and questionnaires presented to physicians and nurses working in intensive care have confirmed poor compliance (7,8).

Full table
The Institute of Health Improvement (IHI) developed the concept of “bundles” applied to the project “Idealized Design of the Intensive Care Unit” (IDICU) in 2001 (9). The new strategy consists of selecting among dozens of preventive measures proposed in guidelines and recommendations of healthcare agencies and/or scientific societies a group of 3 to 5 measures, or “bundle”, of only those interventions with the highest, level 1, evidence and ease of implementation, which, when applied simultaneously, are more effective than if used separately. Selecting only a small number of measures facilitates education, has a higher potential to achieve adequate adherence and to be accessible to audit. The two first bundles to be developed were the IHI Central Line Bundle (hand hygiene, maximal barrier precautions, chlorhexidine skin antisepsis, optimal catheter site selection, avoidance of the femoral vein for central venous access in adult patients, and daily review of line necessity, with prompt removal of unnecessary lines) and the IHI Ventilator Bundle (elevation of the head of the bed to 30 to 45 degrees, daily “sedation vacations” and assessment of readiness to extubate, peptic ulcer disease prophylaxis and deep venous thrombosis prophylaxis). The multimodal strategy for implementation of the IHI Central Line Bundle in ICUs in the State of Michigan was associated with a spectacular reduction in catheter-related bloodstream infection (CRBSI) and the development of the concept of “Zero Bacteremia” (10). The implementation of these bundles improved healthcare quality, particularly in patients at increased risk, by improving the process of care and the behavior of HCW. The Michigan initiative has been replicated with success in other countries, where a similar methodology was used.
Background of the multimodal VAP preventions strategy
In 2002 the Agency for Healthcare Research and Quality and the National Quality Forum launched “The 100k lives campaign”, including the IHI Ventilator Bundle, with two of the proposed measures being specific for the prevention of VAP (11). In 2005, Resar et al. (12) reported on the impact of applying the IHI Ventilator Bundle in 35 hospitals in the USA, with those achieving >95% compliance experiencing a reduction of 59% in VAP rate (from 6.6 to 2.7 episodes per 1,000 days of mechanical ventilation). Similar observations with bundles used in single center (13-32) or small multicenter (33-38) studies have been published, all observing important decreases of VAP rates. In some of these, the effect was only transient and had no impact on other indicators like duration of mechanical ventilation, ICU length-of-stay or mortality. Also, nation-wide implementation of bundles is associated with significant reduction in VAP rates (39-44) (Table 2). A recent meta-analysis (45) includes randomized and non-randomized studies published before June 2017, using VAP prevention bundles and reporting on their effect on mortality. Its results show that “simple interventions in common clinical practice applied in a coordinated way as a part of a bundle care are effective in reducing mortality in ventilated ICU patients.”
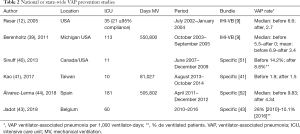
Full table
Another of these nation-wide projects was developed in Spain. Device-associated Spanish ICU-acquired infection rates are well known since 1994 through the “Estudio Nacional de Vigilancia de Infección Nosocomial” (ENVIN-HELICS) registry, where clinical data of patients admitted to ICUs in Spain yearly between April and June are collected (46). VAP rates had remained stable nationally from 2000 to 2008 at around 15 episodes per 1,000 days of mechanical ventilation (46,47), much higher compared to the USA “National Healthcare Safety Networt” (NHSN) registry, where rates from 2006 to 2008 are 2.1 episodes in adult mixed or pediatric ICUs and 10.7 episodes per 1,000 days of mechanical ventilation in burn units (48). From 2009 to 2010 the Project “Bacteriemia Zero” (BZ) was developed to apply a bundle of measures aiming at preventing CRBSI. Zero Bacteremia achieved a reduction of national rates of 50% (49), and an associated decrease of VAP rates to 11.5 episodes per 1,000 days of mechanical ventilation, a “collateral benefit” attributed to promoting interventions improving hygiene (46). Taking advantage of the organizational structure of BZ, a new project was designed in 2011–2012, named “Neumonía Zero” (NZ) or “Zero VAP”, to be implemented nationally. The NZ bundle was composed of specific preventive interventions and associated with a higher than 50% reduction of VAP rates (from 9.83 episodes per 1,000 ventilator days in the baseline period to 4.34 after 19–21 months of participation) which persists nowadays, several years after the implementation period (43,46). Experience gathered with this project has allowed to define the core elements for the success associated with the nation-wide application of NZ: (I) promotion of the project by healthcare authorities and scientific societies, (II) selection of the evidence-based recommendations of the bundle, (III) implementation of the project in ICUs, (IV) education of HCW in a culture of patient safety, (V) audits of compliance with recommendations, (VI) commitment of hospital management to support and facilitate application of bundles, (VII) nomination and empowerment of local ICU leaders (physicians and nurses) and (VIII) a registry continuously collecting VAP rates and providing information to HCW and authorities.
Conditions required for the successful implementation of a national VAP prevention bundle
The publication of a bundle of recommendations for the prevention of a particular healthcare-associated infection does not guarantee its application, nor its success in reducing incidence rates (27,50). A project aiming at nation-wide implementation of the recommendations of a VAP prevention bundle needs to consider and deal with the difficulties of the following development phases to achieve success.
Promotion of the project by healthcare authorities and scientific societies
Healthcare organizations of different types, supported by scientific societies, have sponsored bundles to be implemented nation-wide, like the Agency for Healthcare Research and Quality and the National Quality Forum in the USA (9), the Federal Service Public in Belgium (43), or the Centers for Disease Control of Taiwan (Taiwan CDC) and the Infection Control Society of Taiwan (41). In Spain, the “Neumonía Zero” project was supported by the Spanish Ministry of Health and co-financed by regional Healthcare Authorities. The Spanish Society of Critical Care Medicine (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias, SEMICYUC) and the Spanish Society of Critical Care Nursing (Sociedad Española de Enfermería Intensiva y Unidades Coronarias, SEEIUC) were in charge of technical coordination of the project through a collaboration contract (44). In every ICU, project leaders were nominated, which included at least one intensivist and a critical care nurse (Figure 1). Participation of ICUs in “Zero VAP” was voluntary and no financial incentives existed for its implementation, although in most regions the objectives of the project were included in the contracts of the Healthcare Authority with hospitals.

Selection of recommendations for the bundle
Many interventions for the prevention of VAP, aiming at different pathophysiologic mechanisms of VAP, have been proposed. The first studies were based on adherence to the recommendations of the bundle proposed by the Agency for Healthcare Research and Quality (9). More recently, multidisciplinary working groups were established, which applied evidence-based methodology to select those recommendations appropriate for the targeted ICUs (34-44,51,52). In Spain, a task force composed of expert intensivists of different working groups of SEMICYUC, as well as representatives of the SEEIUC, was in charge of selecting preventive measures for the VAP bundle (52). A systematic and iterative review of the literature and the recommendations of scientific societies and expert groups identified 35 specific VAP prevention interventions (Table 1), which were classified as functional (15), mechanical (13) or pharmacological (7). Each type of measures was subsequently studied separately by at least two members of the task force, who reviewed the respective clinical trials. Quality of the available evidence was established according to GRADE (Grading of Recommendations Assessment, Development and Evaluation Working Group) methodology (53). Finally, in a second step, those recommendations considered more effective, tolerable and feasible were selected as mandatory for participation in “Neumonía Zero”, the National Program for VAP prevention. In addition, other non-mandatory, although highly recommended, interventions were proposed (Table 3) for nation-wide application.
Implementation of the project
Bundles may be implemented either through a nation-wide proposal by the healthcare authorities or by an initiative of HCW at one or several ICUs. For both approaches, success requires physician and nurse leaders at each ICU, capable of promoting education, adherence to recommendations and continuous registry of infections. Conversely, only publishing a bundle of recommendations is no guarantee of its application. Different implementation strategies have been used, all being multifaceted interventions and including educational meetings (a standardized presentation delivered in electronic format), supportive material like posters, and multidisciplinary meetings and workshops with leaders from participating ICUs. In a systematic review carried out to determine the strategies used to implement the bundles, it was observed that the three most frequent used were education, reminders and audit and feedback (54). Hawe et al. (27) reported a significant reduction of VAP rates, from 19.2 to 7.5 per 1,000 of mechanical ventilation if a VAP prevention bundle was actively associated with a multimodal program incorporating staff education, process measurement and outcome measurement, as well as feedback to staff and organizational change.
Education of healthcare workers in patient safety applied to VAP prevention
Both physicians and nurses working in critical care are requested to attend educational courses including at least information about the impact of VAP and the evidence background of the selected recommendations for its prevention. Some studies have shown significant reductions of VAP when multidisciplinary structured educational programs were delivered to ICU HCW in charge of ventilated patients (55,56), although simple communication of knowledge may be insufficient to modify behavior (57). Conveying the concept that VAP is an adverse event and that everybody involved in patient care is responsible for its occurrence is an essential element to promote adherence to recommendations. Audit of adherence, identification of errors in clinical practice, transfer of daily information, communication of adverse events and the objectives of improvement initiatives are tools reinforcing good clinical practice. Project NZ developed online education (available online: http://hws.vhebron.net/neumonia-zero/Nzero.asp) for participating healthcare personnel, which included specific content for application of the recommendations, as well as patient safety assurance information. Furthermore, the number of staff completing the 6-hour online training course was recorded.
Monitoring of adherence
The degree of adherence to recommendations for VAP prevention is the main factor associated with success. Cocanour et al. (22) demonstrated that in critically ill trauma patients VAP rates only decreased after introducing daily audit of adherence and establishing weekly communication of degree of adherence. Engagement of the hospital management, together with education and information about results to HCW was decisive in reducing VAP rates. Bird et al. (21) observed a progressive reduction of VAP in two surgical ICUs as a function of increasing adherence to the bundle. Bonello et al. (58) report that an increase in adherence of the IHI Ventilator Bundle from 50% to 82% was associated with a 41% reduction in VAP rate in 9 ICU Departments of Veterans Affairs Hospitals. In our Project, adherence was measured monitoring the monthly collection of data in the online data base by the participating ICUs, which is required for the calculation of rates (episodes of VAP, length of stay, days on ventilator), internal quality audits of four of the recommendations (measuring cuff pressure, oral hygiene with chlorhexidine, tracheal secretion suctioning and head rest elevation) and a survey asking for the preventive measures active in each ICU.
Commitment of hospital management to support the application of bundles
The 5 Million Lives Campaign was launched in conjunction with IHI’s National Forum meeting in December 2006 (59). The new recommendations “…target Governance structures (which can have enormous impact on a facility’s ability to drive change)”. Although there are no data available about the impact of the participation of hospital managers, we are convinced that the implementation of bundles in an ICU must be preceded by the commitment of its hospital management to support the strategies that have been shown to be effective in reducing VAP. This commitment includes taking responsibility in the project by facilitating the redistribution of resources, guarantee training sessions and keeping record of the proposed activities, empower physician and nurse leaders of the ICU to audit and guarantee adherence to recommendations, participate in meetings of the local project team, which include monitoring of adherence, and feed-back session where results are presented. The presence of hospital managers provides strength to the application of recommendations and expresses their commitment. Implementation of bundles to optimize VAP prevention is not a clinical study performed during a given period of time by a group of investigators to demonstrate the efficacy of the preventive measures, but an objective of hospital managers to favor change of behavior and the culture of patient safety of HCW, particularly in the ICU, to introduce recommendations of the highest degree of evidence in clinical practice.
Identification and empowerment of leader of the project in the ICU
Successful VAP prevention, as avoiding any other infection or adverse event, depends on the existence of leaders of the project in every ICU. Prevention of VAP is a shared responsibility of physicians and nurses, who should clearly identify their leaders, thus facilitating the formation of working groups to apply recommendations, train HCW, audit adherence and register infectious episodes. They also participate in meetings with the hospital management and collaborate in identifying errors in clinical practice and establishing goals for improvement. Finally, local leaders should regularly provide information to HCW about infection rates and the results of the meetings with the hospital management.
Registry of VAP episodes—data collection and measures
To evaluate the impact of the bundle, the definition of VAP needs to be agreed upon and the registry collecting this information should use the very same definition. The diagnosis of VAP ideally follows definitions used by surveillance systems of healthcare related infections. Workshops standardizing the use of definitions in doubtful cases reduce variability and bias. Rates are expressed in most studies as episodes per 1,000 days of mechanical ventilation, with continuous evaluation of the impact of the prevention program. Some have used means or medians in studies with a before-after design of the application of bundles. Others have expressed data in percentage of VAP episodes per 100 mechanically ventilated patients. We used the European Center for Disease Control (ECDC) criteria for the diagnosis of VAP (60) and workshops were held during the implementation period of the Project to standardize definitions. Online data collection was continuous on a specific adaptation of the ENVIN-HELICS registry (http://hws.vhebron.net/Neumonia-zero/), with restricted access and on a voluntary basis. Every ICU reported monthly on number of new admissions, length of stay and duration of mechanical ventilation. They also had access to continuously updated results of its own rates, its region’s results, as well as the simultaneous national rates over the entire duration of the project and beyond. In the Belgian National Study, VAP prevalence rates were determined on one single day by means of a questionnaire completed at each ICU, including the number of patients on mechanical ventilation and patients diagnosed of VAP. Rates were expressed as VAP cases per 100 ventilated patients.
Strength and barriers of VAP prevention bundles
A nation-wide application of VAP prevention bundles requires an organizational structure, which is participatory and transversal, including regional and national healthcare organizations, scientific societies, hospital management, infection control staff, physician and nurse leaders in ICUs, and the collaboration of all HCW working in the ICU. The greater the implication of each category in the pyramid of liabilities, the bigger is the chance of success. The main barrier to implementation is uncoordinated activity of participants. Involved parties need to work in coordination, unconditionally and avoiding egos. Some units argued that the need for additional resources to apply the recommendations and to collect the data needed for calculation of rates is a barrier for participation in the National Projects and for implementing the bundles.
The training of HCW in charge of the critically ill in applying the VAP bundle is associated with conveying the concept of patient safety and the use of tools like identification of errors, communication, meetings with hospital management and establishing targets for improvement. In Spain, the bundles for the prevention of CRBSI and VAP have been key to facilitate the dissemination of a culture of critically ill patient safety.
Most studies show that the application of VAP bundles is associated with significant reductions in VAP rates. This impact grows as a function of time and adherence. The low VAP rates reached have persisted, as indicators of national and international registries of this infection reveal (1,61-63). As a consequence, quality reference standards for VAP rates have been adapted. In the Calgary Health Region (Canada), ICUs reduced their VAP rate goal in 2008 from 9.9 to 7.4 cases per 1,000 ventilator-days after incorporating a prevention bundle (34). In Spain, SEMICYUC has reduced the reference standard quality indicator for VAP from 18 episodes in 2005 to 12 in 2011 and to 7 in 2017 per 1,000 days of mechanical ventilation, after “Neumonía Zero” (4).
Limitations of VAP prevention bundle studies
The results of research on the impact of VAP bundles are hampered by their design, because they are neither randomized, nor blinded, nor do they have parallel control groups (64). All of them evaluate the impact of bundles over time, i.e., a before-after design, with the argument that randomization would be unethical because it is well known that bundles improve outcome. Another source of bias is the identification of VAP, if alternative diagnoses like “tracheobronchitis” or “mechanical ventilation-related respiratory tract infection” are used. Some have expressed their concern that small modifications of diagnostic criteria may have a big impact on rates. This may actually occur in a single ICU, but is probably not relevant in a nation-wide intervention with participation of over 200 units and a huge number of days of observation.
The results of single center studies are difficult to interpret because they don’t report on adherence rate, don’t control for other VAP specific risk factors, and use criteria for the diagnosis of VAP different from national and international surveillance studies (1,61,62). These apply robust methods to collect data, including associated risk factors for VAP and specific definitions, and confirm a progressive reduction of the rates in those countries where programs have been introduced nation-wide for the implementation of bundles.
National studies about VAP bundles have used several data collection methods, some of which may be biased. Incomplete or incorrect data collection may increase if the process is not closely monitored. In addition, the participation of multiple observers increases the risk of diagnostic error. Some national studies have held workshops to standardize the definition of VAP and external audits of cases entered in the national registry.
Conclusions
Local and national VAP prevention bundles have shown to be efficient in transferring knowledge to clinical practice in ICUs. The strategies used most frequently for implementation of VAP bundles have been education, posters, audits of adherence, and feedback of results. Most initiatives have accomplished significant reduction in VAP rates, in spite of considerable heterogeneity of recommendations, ways of implementation, auditioning of adherence and registry of VAP rates.
Acknowledgements
To all the leaders of the “Pneumonia Zero project” in the participating ICU. To intensivist doctors and nurses who work in the ICU participants who have followed the recommendations of the “Pneumonia Zero project”.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- European Centre for Disease Prevention and Control. Healthcare-associated infections acquired in intensive care units. Reporting on data retrieved TERRy on 22 May 2017. Available online: https://ecde.europa.eu/en/publications-data
- Dudeck MA, Edwards JR, Allen-Bridson K, et al. National Healthcare Safety Network report, data summary for 2013, Device-associated Module. Am J Infect Control 2015;43:206-21. [Crossref] [PubMed]
- Klompas M, Kleinman K, Murphy MV. Descriptive epidemiology and attributable morbidity of ventilator-associated events. Infect Control Hosp Epidemiol 2014;35:502-10. [Crossref] [PubMed]
- Sociedad Española de Medicina Intensiva Crítica y Unidades Coronarias (SEMICYUC). Indicadores de calidad en el enfermo crítico. Actualización del 2017. Available online: http://www.semicyuc.org/sites/default/files/indicadoresCalidad2017/INDICADORESDECALIDAD2017_SEMICYUC.pdf
- Rello J, Lode H, Cornaglia G, et al. A European bundle for prevention of ventilator associated pneumonia. Intensive Care Med 2010;36:773-80. [Crossref] [PubMed]
- Hellyer TP, Ewan V, Wilson P, et al. The Intensive Care Society recommended bundle of interventions for the prevention of ventilator-associated pneumonia. J Intensive Care Soc 2016;17:238-43. [Crossref] [PubMed]
- Labeau S, Vandijck D, Rello J, et al. Evidence-based guidelines for the prevention of ventilator-associated pneumonia; results of a knowledge test among European intensive care nurses. J Hosp Infect 2008;70:180-5. [Crossref] [PubMed]
- Lambert ML, Palomar M, Agodi A, et al. Prevention of ventilator-associated pneumonia in intensive care units: an international online survey. Antimicrob Resist Infect Control 2013;2:9. [Crossref] [PubMed]
- Resar R, Griffin FA, Haraden C, et al. Using Care Bundles to Improve Health Care Quality. IHI Innovation Series white paper. Cambridge, Massachusetts: Institute for Healthcare Improvement, 2012.
- Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006;355:2725-32. [Crossref] [PubMed]
- Berwick DM, Calkins DR, McCannon CJ, et al. The 100 000 Lives Campaign Setting a Goal and a Deadline for Improving Health Care Quality. JAMA 2006;295:324-7. [Crossref] [PubMed]
- Resar R, Pronovost P, Haraden C, et al. Using a bundle approach to improve ventilator care processes and reduce ventilator-associated pneumonia. Jt Comm J Qual Patient Saf 2005;31:243-8. [Crossref] [PubMed]
- Youngquist P, Carroll M, Farber M, et al. Implementing a ventilator bundle in a community hospital. Jt Comm J Qual Patient Saf 2007;33:219-25. [Crossref] [PubMed]
- Unahalekhaka A, Jamulitrat S, Chongsuvivatwong V, et al. Using a collaborative to reduce ventilator-associated pneumonia in Thailand. Jt Comm J Qual Patient Saf 2007;33:387-94. [Crossref] [PubMed]
- Berriel-Cass D, Adkins FW, Jones P, et al. Eliminating nosocomial infections at Ascension Health. Jt Comm J Qual Patient Saf 2006;32:612-20. [Crossref] [PubMed]
- Apisarnthanarak A, Pinitchai U, Thongphubeth K, et al. Effectiveness of an educational program to reduce ventilator-associated pneumonia in a tertiary care center in Thailand: a 4-year study. Clin Infect Dis 2007;45:704-11. [Crossref] [PubMed]
- Baxter AD, Allan J, Bedard J, et al. Adherence to simple and effective measures reduces the incidence of ventilator-associated pneumonia. Can J Anaesth 2005;52:535-41. [Crossref] [PubMed]
- Omrane R, Eid J, Perreault MM, et al. Impact of a protocol for prevention of ventilator-associated pneumonia. Ann Pharmacother 2007;41:1390-6. [Crossref] [PubMed]
- Zaydfudim V, Dossett LA, Starmer JM, et al. Implementation of a real-time compliance dashboard to help reduce SICU ventilator-associated pneumonia with the ventilator bundle. Arch Surg 2009;144:656-62. [Crossref] [PubMed]
- Blamoun J, Alfakir M, Rella ME, et al. Efficacy of an expanded ventilator bundle for the reduction of ventilator-associated pneumonia in the medical intensive care unit. Am J Infect Control 2009;37:172-5. [Crossref] [PubMed]
- Bird D, Zambuto A, O'Donnell C, et al. Adherence to ventilator-associated pneumonia bundle and incidence of ventilator-associated pneumonia in the surgical intensive care unit. Arch Surg 2010;145:465-70. [Crossref] [PubMed]
- Cocanour CS, Peninger M, Domonoske BD, et al. Decreasing ventilator-associated pneumonia in a trauma ICU. J Trauma 2006;61:122-9. [Crossref] [PubMed]
- Jain M, Miller L, Belt D, et al. Decline in ICU adverse events, nosocomial infections and cost through a quality improvement initiative focusing on teamwork and culture change. Qual Saf Health Care 2006;15:235-9. [Crossref] [PubMed]
- Dubose J, Teixeira PG, Inaba K, et al. Measurable outcomes of quality improvement using a daily quality rounds checklist: one-year analysis in a trauma intensive care unit with sustained ventilator-associated pneumonia reduction. J Trauma 2010;69:855-60. [Crossref] [PubMed]
- Al-Thaqafy MS, El-Saed A, Arabi YM, et al. Association of compliance of ventilator bundle with incidence of ventilator-associated pneumonia and ventilator utilization among critical patients over 4 years. Ann Thorac Med 2014;9:221-6. [Crossref] [PubMed]
- Khan R, Al-Dorzi HM, Al-Attas K, et al. The impact of implementing multifaceted interventions on the prevention of ventilator-associated pneumonia. Am J Infect Control 2016;44:320-6. [Crossref] [PubMed]
- Hawe CS, Ellis KS, Cairns CJ, et al. Reduction of ventilator-associated pneumonia: active versus passive guideline implementation. Intensive Care Med 2009;35:1180-6. [Crossref] [PubMed]
- Bouadma L, Deslandes E, Lolom I, et al. Long-term impact of a multifaceted prevention program on ventilator-associated pneumonia in a medical intensive care unit. Clin Infect Dis 2010;51:1115-22. [Crossref] [PubMed]
- Sen S, Johnston C, Greenhalgh D, et al. Ventilator-Associated Pneumonia Prevention Bundle Significantly Reduces the Risk of Ventilator-Associated Pneumonia in Critically Ill Burn Patients. J Burn Care Res 2016;37:166-71. [Crossref] [PubMed]
- Morris AC, Hay AW, Swann DG, et al. Reducing ventilator-associated pneumonia in intensive care: Impact of implementing a care bundle. Crit Care Med 2011;39:2218-24. [Crossref] [PubMed]
- O'Horo JC, Lan H, Thongprayoon C, et al. "Bundle" Practices and Ventilator-Associated Events: Not Enough. Infect Control Hosp Epidemiol 2016;37:1453-7. [Crossref] [PubMed]
- Talbot TR, Carr D, Parmley CL, et al. Sustained Reduction of Ventilator-Associated Pneumonia Rates Using Real-Time Course Correction With a Ventilator Bundle Compliance Dashboard. Infect Control Hosp Epidemiol 2015;36:1261-7. [Crossref] [PubMed]
- Rosenthal VD, Guzman S, Crnich C. Impact of an infection control program on rates of ventilator-associated pneumonia in intensive care units in 2 Argentinean hospitals. Am J Infect Control 2006;34:58-63. [Crossref] [PubMed]
- Esmail R, Duchscherer G, Giesbrecht J, et al. Prevention of ventilator-associated pneumonia in the Calgary Health region: a Canadian success story! Healthc Q 2008;11:129-36. [Crossref] [PubMed]
- Eom JS, Lee MS, Chun HK, et al. The impact of a ventilator bundle on preventing ventilator-associated pneumonia: a multicenter study. Am J Infect Control 2014;42:34-7. [Crossref] [PubMed]
- Lim KP, Kuo SW, Ko WJ, et al. Efficacy of ventilator-associated pneumonia care bundle for prevention of ventilator-associated pneumonia in the surgical intensive care units of a medical center. J Microbiol Immunol Infect 2015;48:316-21. [Crossref] [PubMed]
- Rosenthal VD, Desse J, Maurizi DM, et al. Impact of the International Nosocomial Infection Control Consortium's multidimensional approach on rates of ventilator-associated pneumonia in 14 intensive care units in 11 hospitals of 5 cities within Argentina. Am J Infect Control 2018;46:674-9. [Crossref] [PubMed]
- Al-Abdely HM, Khidir Mohammed Y, Rosenthal VD, et al. Impact of the International Nosocomial Infection Control Consortium (INICC)'s multidimensional approach on rates of ventilator-associated pneumonia in intensive care units in 22 hospitals of 14 cities of the Kingdom of Saudi Arabia. J Infect Public Health 2018;11:677-84. [Crossref] [PubMed]
- Berenholtz SM, Pham JC, Thompson DA, et al. Collaborative cohort study of an intervention to reduce ventilator-associated pneumonia in the intensive care unit. Infect Control Hosp Epidemiol 2011;32:305-14. [Crossref] [PubMed]
- Sinuff T, Muscedere J, Cook D, et al. Implementation of clinical practice guidelines for ventilator-associated pneumonia: a multicenter prospective study. Crit Care Med 2013;41:15-23. [Crossref] [PubMed]
- Kao CC, Chiang HT, Chen CY, et al. National bundle care program implementation to reduce ventilator-associated pneumonia in intensive care units in Taiwan. J Microbiol Immunol Infect 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Apisarnthanarak A, Ratz D, Greene MT, et al. National survey of practices to prevent health care-associated infections in Thailand: The role of prevention bundles. Am J Infect Control 2017;45:805-10. [Crossref] [PubMed]
- Jadot L, Huyghens L, De Jaeger A, et al. Impact of a VAP bundle in Belgian intensive care units. Ann Intensive Care 2018;8:65. [Crossref] [PubMed]
- Álvarez-Lerma F, Palomar-Martínez M, Sánchez-García M, et al. Prevention of Ventilator-Associated Pneumonia: The Multimodal Approach of the Spanish ICU "Pneumonia Zero" Program. Crit Care Med 2018;46:181-8. [Crossref] [PubMed]
- Pileggi C, Mascaro V, Bianco A, et al. Ventilator Bundle and Its Effects on Mortality Among ICU Patients: A Meta-Analysis. Crit Care Med 2018;46:1167-74. [Crossref] [PubMed]
- Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias (SEMICYUC). Estudio Nacional de Vigilancia de Infecciones Nosocomiales (ENVIN). Available online: http://hws.vhebron.net/envin-helics/
- Alvarez-Lerma F, Palomar M, Olaechea P, et al. Grupo de Estudio de Vigilancia de Infección Nosocomial en UCI. National Study of Control of Nosocomial Infection in Intensive Care Units. Evolutive report of the years 2003-2005. Med Intensiva 2007;31:6-17. [Crossref] [PubMed]
- Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am J Infect Control 2009;37:783-805. [Crossref] [PubMed]
- Palomar M, Álvarez-Lerma F, Riera A, et al. Impact of a national multimodal intervention to prevent catheter-related bloodstream infection in the ICU: the Spanish experience. Crit Care Med 2013;41:2364-72. [Crossref] [PubMed]
- Furuya EY, Dick A, Perencevich EN, et al. Central line bundle implementation in US intensive care units and impact on bloodstream infections. PLoS One 2011;6:e15452. [Crossref] [PubMed]
- Muscedere J, Dodek P, Keenan S, et al. Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia. Prevention. J Crit Care 2008;23:126-37. [Crossref] [PubMed]
- Álvarez Lerma F, Sánchez García M, Lorente L, et al. Guidelines for the prevention of ventilator-associated pneumonia and their implementation. The Spanish "Zero-VAP" bundle. Med Intensiva 2014;38:226-36. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Borgert MJ, Goossens A, Dongelmans DA. What are effective strategies for the implementation of care bundles on ICUs: a systematic review. Implement Sci 2015;10:119. [Crossref] [PubMed]
- Babcock HM, Zack JE, Garrison T, et al. An educational intervention to reduce ventilator-associated pneumonia in an integrated health system: a comparison of effects. Chest 2004;125:2224-31. [Crossref] [PubMed]
- Salahuddin N, Zafar A, Sukhyani L, et al. Reducing ventilator-associated pneumonia rates through a staff education programme. J Hosp Infect 2004;57:223-7. [Crossref] [PubMed]
- Sinuff T, Muscedere J, Adhikari NK, et al. Knowledge translation interventions for critically ill patients: a systematic review. Crit Care Med 2013;41:2627-40. [Crossref] [PubMed]
- Bonello RS, Fletcher CE, Becker WK, et al. An intensive care unit quality improvement collaborative in nine Department of Veterans Affairs hospitals: reducing ventilator-associated pneumonia and catheter-related bloodstream infection rates. Jt Comm J Qual Patient Saf 2008;34:639-45. [Crossref] [PubMed]
- Institute for Healthcare Improvement. 5 million lives campaign. Available online: http://www.ihi.org/about/Documents/5MillionLivesCampaignCaseStatement.pdf
- European Centre for Disease Prevention and Control. European surveillance of healthcare-associated infections in intensive care units. HAI-Net ICU protocol, version 1.02. Stockholm: ECDC; 2015. Available online: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/healthcare-associated-infections-HAI-ICU-protocol.pdf
- Rosenthal VD, Rodrigues C, Álvarez-Moreno C, et al. Effectiveness of a multidimensional approach for prevention of ventilator-associated pneumonia in adult intensive care units from 14 developing countries of four continents: findings of the International Nosocomial Infection Control Consortium. Crit Care Med 2012;40:3121-8. [Crossref] [PubMed]
- Rosenthal VD, Al-Abdely HM, El-Kholy AA, et al. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010-2015: Device-associated module. Am J Infect Control 2016;44:1495-504. [Crossref] [PubMed]
- Reper P, Dicker D, Damas P, et al. Improving the quality of the intensive care follow-up of ventilated patients during a national registration program. Public Health 2017;148:159-66. [Crossref] [PubMed]
- Zilberberg MD, Shorr AF, Kollef MH. Implementing quality improvements in the intensive care unit: ventilator bundle as an example. Crit Care Med 2009;37:305-9. [Crossref] [PubMed]



