Surgical antimicrobial prophylaxis in intensive care unit (ICU) patients: a preliminary, observational, retrospective study
Introduction
The use of surgical antimicrobial prophylaxis (SAP) in the operating room is supported by evidence-based guidelines (1). The goal of SAP is to decrease surgical site infection (SSI) rates (2,3). The rules for prescription of SAP are stringent: the antimicrobial drug must be a narrow-spectrum agent (1), the drug should be administered 30–60 minutes before surgical incision (4-6) and the duration of prophylactic treatment should not exceed 48 h (7,8).
Nevertheless, SAP guidelines do not cover all clinical scenarios and the efficacy of SAP depends on several factors, including selection of the appropriate antibiotic(s), the timing of antibiotic administration, its dosage, and route of administration. Patients admitted to an intensive care unit (ICU) are often exposed to broad-spectrum antibiotics, multi-drug resistant (MDR) pathogen carriage and recurrent infections (9). More than 75% of them receive at least one antimicrobial drug during their ICU stay (10). They are also exposed to endogenous (organ failure, immunosuppression) and exogenous (invasive devices) factors which increase the risk of healthcare-associated infection. In France, the RAISIN network showed that 10.6% of extended-spectrum beta-lactamase-producing Enterobacteriaceae (ESBL-E) and 9.3% of methicillin-resistant Staphylococcus aureus (MRSA) contaminations were found in ICU patients. In addition, around 11% of patients hospitalized more than 2 days in ICU were colonized by MDR pathogens (9,11).
To our knowledge, no information is available SAP in patients who recently received an antimicrobial therapy, in those with bacterial colonization, or in those at higher risk of carrying MDR pathogens. ICU patients may require transfer to the operating room for surgery, thereby requiring SAP. To assess this issue, we designed a retrospective study, with a primary objective of describing our professional practices in ICU patients requiring SAP. The secondary objectives were to report the incidence and ecology of postoperative infections in our ICU patients.
Methods
Study design and study participants
This descriptive, non-interventional, single-center, retrospective study was carried out in the department of Anesthesiology and Critical Care Medicine of the Teaching Hospital “Hôpital Nord” in Marseille, France, from January 1st to December 31st, 2016. Included were all adult patients admitted to the ICU and scheduled for surgical intervention in the operating room following at least 48 hours of ICU stay. We excluded from our analysis the patients with incomplete data, those in brain death undergoing organ donation and those dying during the surgery. Since our study was retrospective and non-interventional, ethical research committee oversight and patient consent were waived (IRB n°00010254-2016-126).
Clinical setting: in our 15-bed ICU, selective digestive tract decontamination is routinely administered only to severe trauma, brain trauma and cardiac arrest patients. Our patients with MRSA carriage or ESBL-E were isolated during the ICU stay. Cultures are not taken routinely for surveillance.
Data collection
Data were retrieved from medical files and clinical software used in our institution. For each patient, the following data were collected:
Preoperative data
- Preoperative colonization: pathogens, antimicrobial susceptibility, sites;
- Preoperative infections: pathogens, antimicrobial susceptibility, sites, antimicrobial therapy used;
- Administration of a preoperative empirical antimicrobial therapy.
- Screening for ESBL-E and MRSA with rectal and/or nasal swab respectively;
- Administration of selective digestive tract decontamination. This is defined in our center as:
- Two grams of intravenous cefazolin every 8 hours for 48 hours and
- Colistin, gentamicin and amphotericin B administered as an oral paste for the duration of mechanical ventilation.
Pathogens are grouped in the results as Gram-negative bacteria, Gram-positive cocci and non-fermenting Gram-negative bacteria to facilitate presentation.
Perioperative and postoperative data
- Type of surgery;
- Surgical wound classification (12);
- Antimicrobial drug (type) used in the operating room;
- Diagnostic of infection and/or colonization within 30 days after surgery;
- Site, microorganisms and drug resistance profile.
Definitions of pre and postoperative infection, colonization and drug resistance profiles:
Preoperative infection was diagnosed using criteria from the international sepsis forum consensus conference (13,14). Preoperative colonization was defined as a positive microbiological sampling without infection criteria and without antimicrobial therapy needed. Microorganisms were classified by their drug-resistance profile using criteria from the REA-RAISIN network (11):
- RAISIN 0: natural resistance profile;
- RAISIN 1: low level of drug resistance (i.e., penicillinase secretion), MRSA susceptible to aminoglycosides;
- RAISIN 2: high level of drug resistance including cephalosporinase secretion, ceftazidime resistance for Gram-negative bacteria, MRSA resistant to aminoglycosides;
- RAISIN 3: multidrug resistance with broader resistance profile than RAISIN 2.
Pathogens with drug-resistance profiles RAISIN 0 and 1 were considered “low level of antimicrobial resistance”. Pathogens with drug-resistance profiles RAISIN 2 and 3 were considered “high level of antimicrobial resistance”.
Surgical wounds were classified following Center for Disease Control Hospital Infection Control Practices Advisory Committee.
Primary and secondary outcome measures
Our primary objective was to describe our professional practices in ICU patients requiring SAP, measuring the adherence to guidelines. The secondary objectives were to report the incidence and ecology of postoperative infections in our ICU patients.
Data from patient files were analyzed by the authors in order to assess the management of the antimicrobial prophylaxis drug prescription, regarding guideline adherence and preoperative infection anamnesis.
Statistical analysis
Statistical analysis was performed using R software version 3.4.1 (London, UK). Only descriptive analyses were used for the purpose of this study. Qualitative variables are presented as numbers and percents. Quantitative variables are presented as medians and interquartile ranges. The number of patients was not calculated before the study because no data were available in the literature. We performed several subgroup univariate analysis in order to identify characteristics associated with occurrence of colonization and infection after surgery. Comparisons between the continuous data were performed using Student’s t-test and with the chi-square test for categorical variables. All comparisons were two-tailed and P<0.05 was required to exclude the null hypothesis. Thus, we considered the study as exploratory and collected the data for 1 year.
Results
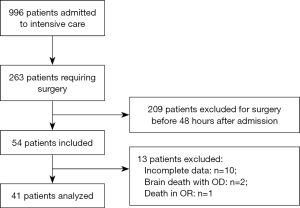
Between January 1st and December 31st of 2016, 996 patients were admitted to the ICU. Among those, 263 (26%) underwent surgery during their ICU stay and 209 were not included because surgery was performed within the first 48 h after ICU admission. Fifty-four patients fulfilled inclusion criteria. Among these 13 were excluded as follows: incomplete data (n=10), brain death with organ donation (n=2), death in the operating room (n=1). The files of 41 patients were analyzed (Figure 1).
SAP management and postoperative impact
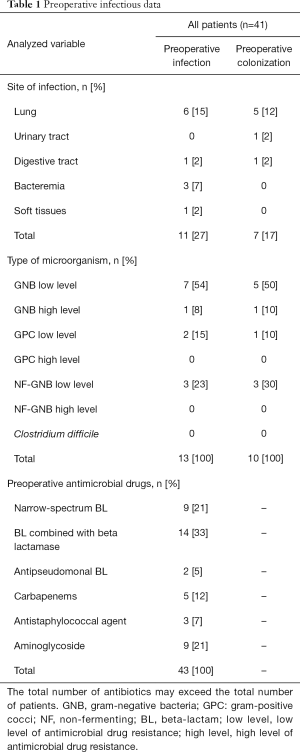
Preoperatively, 13 (32%) patients were being treated for an ongoing infection and 7 (17%) patients were colonized. At least 1 antibiotic was administered before surgery to 21 (51%) patients (Table 1). The surgical wounds were classified as reported above (14): clean, clean/contaminated, contaminated, and dirty in 20 (49%), 7 (17%), 1 (2%) and 13 (32%) of interventions respectively. According to guidelines, SAP was recommended for each patient.
Full table
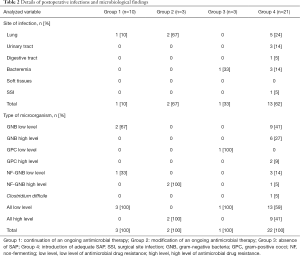
For 10 (24%) patients (group 1), antimicrobial therapy that was already being administered in the ICU (either empirical or tailored to microbiological findings) was also continued in the operating room. Only one patient developed postoperative infection (ventilator-associated pneumonia) due to a Gram-negative bacterium with a low-level of antimicrobial resistance. No SSIs were identified. For 3 (7%) patients (group 2), the antimicrobial therapy given in the ICU was modified during the procedure. One patient received antimicrobial therapy in accordance with guidelines (1) and the 2 others received a combination of meropenem and amikacin. Two of the 3 patients developed postoperative pulmonary infections with highly resistant pathogens (Pseudomonas aeruginosa). Three (7%) patients (group 3) received no SAP in the operating room. One out of these 3 patients developed a bacteremia with a low-resistance pathogen.
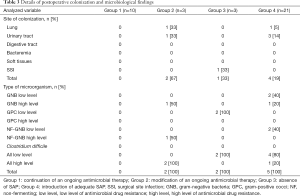
Twenty-one (51%) patients (group 4) required SAP and the drug was chosen according to local guidelines. Thirteen postoperative infections were identified, including 1 SSI due to an ESBL Klebsiella pneumonia and methicillin-resistant Staphylococcus epidermidis. A total of 27 pathogens were found, including 17 (63%) pathogens with low antimicrobial resistance and 10 (37%) with high antimicrobial resistance. Details on postoperative infections are shown in Table 2. Details on postoperative colonization are found in Table 3.
Full table
Full table
We identified 4 (10%) patients with an infectious history that raised issues regarding SAP prescription. The patients were either colonized with resistant pathogens or had recently been treated with antimicrobials that were broader than the spectrum of the recommended SAP drugs. Two patients received SAP in accordance with local guidelines, but the drug chosen provided coverage that was narrower than the antimicrobial treatment administered in the ICU stay or that required to cover existing bacterial colonization. These patients did not develop postoperative infections. Two patients received SAP with a drug not recommended by the local guidelines, but the drug was chosen based on prior antimicrobial treatment during ICU stay or bacterial colonization. These two patients developed urinary tract infections with low-antimicrobial resistance pathogens (Table S1). The details of postoperative infectious data are shown in Table 4.
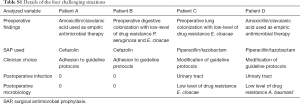
Full table
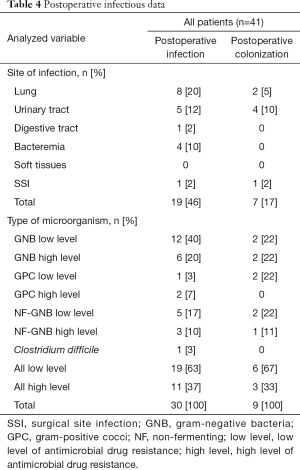
Full table
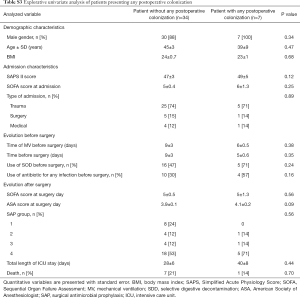
Explorative univariate analysis comparing patients presenting: (I) colonization; (II) infection; (III) both colonization or infection in the 30 days after surgery are presented in Table 5 and in Tables S2,S3.
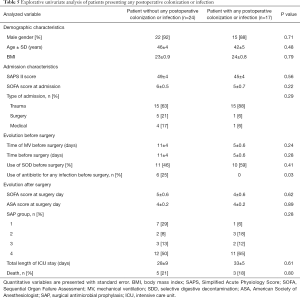
Full table
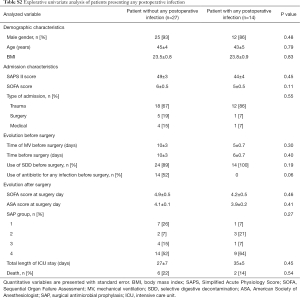
Full table
Full table
Discussion
Our study unveiled heterogeneity in practice of SAP for patients going to surgery more than 48 hours after ICU admission. SAP in intensive care patients is controversial. Current guidelines do not offer definitive answers but do comprise best practice recommendations. The guidelines suggest that when protocols are modified, potential infectious hazards should be clearly identified and the advantage of deviating from recommendations be evaluated against the potential harm such practice may have for the community (1). However, the impact of colonization or ongoing infection due to MDR bacteria on SAP efficacy remains poorly assessed. As a result, in routine practice adherence to this recommendation in ICU patients remains challenging.
Patients receiving antimicrobial therapy before surgery continued the same antibiotic regime in 77% of the cases. Among these, only a single case of postoperative pneumonia was reported. In contrast, we report a high rate of postoperative infection due to resistant pathogens in patients for whom ongoing antimicrobial therapy was modified or discontinued in the operating room. This finding would suggest an advantage for non-modification of ongoing antimicrobial treatment, although future studies are needed to confirm this hypothesis (15,16).
In ICU patients that were not receiving antimicrobial therapy prior to the operating room, adherence to guidelines was feasible in the majority of cases (84%). Among those patients, the rate of SSI observed in the current study (5%) correlates with that described in the general literature (0.1% to 50%) (17). The proportion of highly resistant pathogens found in the current study (38%) was also in line with a previous study (18). These preliminary findings would suggest that SAP guidelines should be followed in ICU patients.
Data from our cohort shows that a clinical documentation of infection or colonization interferes with SAP prescription in only 10% of cases. In this situation, the heterogeneity of SAP management observed in our study underlines the need for large-scale studies and guidelines elaboration.
Our study suffers several limitations. The small number of patients included in the study does not allow definitive conclusions. The retrospective study design and the fact that the data was taken from only one center limits generalization of our results. We do not follow a preset protocol for patients undergoing surgery during their ICU stay. Neither postoperative infections nor colonization can be attributed to surgery or SAP. Results of anal swabbing was not taken into account for SAP prescription. In our unit, we do not perform systematic screening at admission. To our knowledge, no definitive data showed a benefit of systematic ESBL-E screening in terms of outcome (19), even if this issue is still matter of debate (20). No conclusion can be drawn from our data regarding the issue of ESBL-E colonization in ICU patients.
In conclusion, this hypothesis-generating study comprises only a preliminary analysis. Our results raise several points that merit further assessment in large-scale prospective studies. Our data suggests that antimicrobial therapy given in the ICU should be continued in the operating room in patients that have spent more than 48 hours in the ICU. Adherence to guidelines for SAP seems feasible and safe in ICU patients. Most importantly, the heterogeneity of practice observed in a single team underlines the need for further guideline elaboration in this complex population of patients.
Acknowledgements
Data management and analysis were directly supported by the proper funds of the Department of Anesthesiology and Critical Care Medicine, North Hospital, Marseille, France.
Footnote
Conflicts of Interest: M Leone receives fees from Pfizer and MSD for lectures. The other authors have no conflicts of interest to declare.
Ethical Statement: Since our study was retrospective and non-interventional, ethical research committee oversight and patient consent were waived (IRB n°00010254-2016-126).
References
- Société française d’anesthésie réanimation. Antibioprophylaxie en chirurgie et médecine interventionnelle (patients adultes). Annales Françaises d’Anesthésie et de Réanimation 2011;30:168-90.
- Bassetti M, Righi E, Astilean A, et al. Antimicrobial prophylaxis in minor and major surgery. Minerva Anestesiol 2015;81:76-91. [PubMed]
- Nelson RL, Gladman E, Barbateskovic M. Antimicrobial prophylaxis for colorectal surgery. Cochrane Database Syst Rev 2014. [PubMed]
- Weber WP, Mujagic E, Zwahlen M, et al. Timing of surgical antimicrobial prophylaxis: a phase 3 randomised controlled trial. Lancet Infect Dis 2017;17:605-14. [Crossref] [PubMed]
- Duclos G, Zieleskiewicz L, Leone M. Antimicrobial prophylaxis is critical for preventing surgical site infection. J Thorac Dis 2017;9:2826-8. [PubMed]
- de Jonge SW, Gans SL, Atema JJ, et al. Timing of preoperative antibiotic prophylaxis in 54,552 patients and the risk of surgical site infection: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96. [Crossref] [PubMed]
- Mohri Y, Tonouchi H, Kobayashi M, et al. Randomized clinical trial of single- versus multiple-dose antimicrobial prophylaxis in gastric cancer surgery. Br J Surg 2007;94:683-8. [Crossref] [PubMed]
- De Chiara S, Chiumello D, Nicolini R, et al. Prolongation of antibiotic prophylaxis after clean and clean-contaminated surgery and surgical site infection. Minerva Anestesiol 2010;76:413-9. [PubMed]
- Raisin. Surveillance des infections nosocomiales en réanimation adulte. Réseau REA-Raisin, France. Résultats 2015. Santé Publique France, 2017.
- Vincent JL, Rello J, Marshall J, et al. International study of the prevalence and outcomes of infection in intensive care units. JAMA 2009;302:2323-9. [Crossref] [PubMed]
- Jarlier V, Arnaud I. Surveillance des bactéries multiresistantes dans les établissements de santé en France Réseau BMR-Raisin - Résultats 2015. Santé Publique France, 2017.
- Mangram AJ, Horan TC, Pearson ML, et al. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97-132; discussion 96. [Crossref] [PubMed]
- Calandra T, Cohen J. International Sepsis Forum Definition of Infection in the ICU Consensus Conference. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 2005;33:1538-48. [Crossref] [PubMed]
- Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial. Intensive Care Med 2014;40:1399-408. [Crossref] [PubMed]
- Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc 2011;86:156-67. [Crossref] [PubMed]
- Abdul-Aziz MH, Sulaiman H, Mat-Nor MB, et al. Beta-Lactam Infusion in Severe Sepsis (BLISS): a prospective, two-centre, open-labelled randomised controlled trial of continuous versus intermittent beta-lactam infusion in critically ill patients with severe sepsis. Intensive Care Med 2016;42:1535-45. [Crossref] [PubMed]
- Korol E, Johnston K, Waser N, et al. A systematic review of risk factors associated with surgical site infections among surgical patients. PLoS One 2013;8. [Crossref] [PubMed]
- Cohen ME, Salmasian H, Li J, et al. Surgical Antibiotic Prophylaxis and Risk for Postoperative Antibiotic-Resistant Infections. J Am Coll Surg 2017;225:631-638.e3. [Crossref] [PubMed]
- Thouverez M, Talon D, Bertrand X. Control of Enterobacteriaceae producing extended-spectrum beta-lactamase in intensive care units: rectal screening may not be needed in non-epidemic situations. Infect Control Hosp Epidemiol 2004;25:838-41. [Crossref] [PubMed]
- Grohs P, Podglajen I, Guerot E, et al. Assessment of five screening strategies for optimal detection of carriers of third-generation cephalosporin-resistant Enterobacteriaceae in intensive care units using daily sampling. Clin Microbiol Infect 2014;20:O879-86. [Crossref] [PubMed]