
Dasatinib in breast cancer: Src-ing for response in all the wrong kinases
Src is a non-receptor tyrosine kinase that has been associated with carcinogenesis, impairs osteoclast bone resorption, enhances angiogenesis in vivo, and plays a role in the development of breast cancer bone metastases (1-3). Writing in Clinical Breast Cancer, Morris et al. present the results of a single-arm phase II clinical trial evaluating a combination of paclitaxel and dasatinib, a Src inhibitor, in patients with HER2-negative metastatic breast cancer (4). The study was halted early due to slow accrual, but the combination treatment did demonstrate activity in some patients. The objective response rate was 23% and the clinical benefit rate (complete response, partial response, or stable disease) was 43%. The majority of patients (80%) had estrogen-receptor expression, and 68% had bone metastases. Within the cohort, there were a significant percentage of patients that were heavily pre-treated: 30% of patients had received 2 or more lines of endocrine therapy for metastatic breast cancer, 22% of patients had received 2 or more lines of chemotherapy for metastatic breast cancer, and over half of the patients (58%) had previously had treatment with taxanes. There was one patient with triple-negative breast cancer who had a complete response and 7 patients with hormone-receptor positive disease who had partial responses. The investigators did evaluate plasma vascular epidermal growth factor receptor 2 (VEGFR2), plasma N-telopeptide (NTX), and circulating tumor cells as possible biomarkers, but did not identify any correlation between these markers and clinical response.
Interest in Src as a therapeutic target has been evident in the literature for over a decade. Zhang et al. performed a genomic analysis on a large cohort of breast cancer patients and found that a gene expression pattern associated with Src activation was strongly associated with late-onset bone metastases in breast cancer, regardless of breast cancer subtype (5). In addition, Src is an important cross-talk factor between the bone marrow microenvironment and breast cancer cells (5). Specifically, breast cancer cell production of Src leads to osteoclast activation, resorption of bone, and growth of lytic bone metastases through the bone loss (6,7). Preclinical testing of Src inhibitors in breast cancer models has predominantly shown inhibition of bone metastasis formation (1,8); however, in the 4T1 murine mammary carcinoma, dasatinib actually enhanced bone metastasis formation (9).
Overall, the clinical trial data using Src inhibitors in unselected metastatic breast cancer patients has been disappointing (Table 1), but a recent study using Src inhibition in combination with chemotherapy and trastuzumab was more promising and reached an objective response rate of 79% (19). Src activation has been found to be a mechanism of resistance in HER2+ breast cancer (20), and perhaps the combination of Src inhibitors and HER2-targeting therapies is a more promising paradigm. The results of Morris et al. and other trials employing Src inhibitors in HER2− breast cancer populations fail to meet the expected outcomes of better response in patients with bone metastases as well as the triple-negative breast cancer patients established by the pre-clinical studies. It is interesting to note that in Morris et al., on-target biomarkers of Src engagement were no different between responders and non-responders, and as is the case in multiple other trials, the responders are generally hormone-receptor positive.
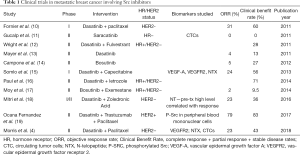
Full table
This leads one to question the role of Src in these patients. Dasatinib is a promiscuous kinase inhibitor and inhibits both the Src tyrosine kinase as well as the ABL tyrosine kinase. It also has effects on the STAT5, c-kit, and platelet-derived growth factor pathways (21). ABL kinase overactivation has been noted in breast cancer cell lines, and has also been found in aromatase-inhibitor resistant breast cancers (22); the PDGFR pathway and c-kit mutations have also been implicated in breast cancer (23).
Although the combination of dasatinib and chemotherapy has in general been lackluster, it is noteworthy that there are two reports of complete responses in the literature with a combination of dasatinib and chemotherapy. One is a recent case report of a patient with simultaneous hormone-receptor positive metastatic breast cancer and chronic myelogenous leukemia (CML) already on dasatinib who developed endocrine therapy-resistance and new liver metastases (24). She received paclitaxel while continuing her dasatinib with stable breast cancer for over a year. At time of liver disease progression, she was switched to vinorelbine + capecitabine with continuation of her dasatinib and achieved a complete response in her visceral disease (stable bone metastases). The other case was presented by Morris et al. in Clinical Breast Cancer: a patient with triple-negative breast cancer with previous taxane-exposure who achieved a complete response with dasatinib and paclitaxel (4).
Exploration of the mechanism of action for these two exceptional responders would be enlightening, as their exquisite response may be related to an alternative pathway inhibited or influenced by dasatinib. For valuable multi-kinase inhibitors such as dasatinib with pleiotropic molecular network interactions, patient-specific functional testing with ex vivo organoids or bespoke patient-derived xenografts may provide a better clue to which breast cancer tumors are most likely to robustly respond.
Acknowledgements
Funding: Dr. Kennedy’s training is supported by the NCI under award number T32CA009515.
Footnote
Conflicts of Interest: Dr. Gadi has the following conflicts of interest: SEngine Precision Medicine (ownership and consulting), Novartis (consulting), Pfizer (consulting), Daichii Sankyo (consulting), Seattle Genetics (consulting), and Genentech (research funding). LC Kennedy has no conflicts of interest to declare.
References
- Rucci N, Recchia I, Angelucci A, et al. Inhibition of protein kinase c-Src reduces the incidence of breast cancer metastases and increases survival in mice: implications for therapy. J Pharmacol Exp Ther 2006;318:161-72. [Crossref] [PubMed]
- Saad F, Lipton A. SRC kinase inhibition: targeting bone metastases and tumor growth in prostate and breast cancer. Cancer Treat Rev 2010;36:177-84. [Crossref] [PubMed]
- Rose AA, Siegel PM. Emerging therapeutic targets in breast cancer bone metastasis. Future Oncol 2010;6:55-74. [Crossref] [PubMed]
- Morris PG, Rota S, Cadoo K, et al. Phase II Study of Paclitaxel and Dasatinib in Metastatic Breast Cancer. Clin Breast Cancer 2018;18:387-94. [Crossref] [PubMed]
- Zhang XH, Wang Q, Gerald W, et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009;16:67-78. [Crossref] [PubMed]
- Clézardin P. Therapeutic targets for bone metastases in breast cancer. Breast Cancer Res 2011;13:207. [Crossref] [PubMed]
- Miyazaki T, Sanjay A, Neff L, et al. Src kinase activity is essential for osteoclast function. J Biol Chem 2004;279:17660-6. [Crossref] [PubMed]
- Zheng MW, Zhang CH, Chen K, et al. Preclinical Evaluation of a Novel Orally Available SRC/Raf/VEGFR2 Inhibitor, SKLB646, in the Treatment of Triple-Negative Breast Cancer. Mol Cancer Ther 2016;15:366-78. [Crossref] [PubMed]
- Hughes VS, Siemann DW. Treatment with Src inhibitor Dasatinib results in elevated metastatic potential in the 4T1 murine mammary carcinoma model. Tumor Microenviron 2018;1:30-6. [Crossref] [PubMed]
- Fornier MN, Morris PG, Abbruzzi A, et al. A phase I study of dasatinib and weekly paclitaxel for metastatic breast cancer. Ann Oncol 2011;22:2575-81. [Crossref] [PubMed]
- Gucalp A, Sparano JA, Caravelli J, et al. Phase II trial of saracatinib (AZD0530), an oral SRC-inhibitor for the treatment of patients with hormone receptor-negative metastatic breast cancer. Clin Breast Cancer 2011;11:306-11. [Crossref] [PubMed]
- Wright G, Blum J, Krekow L, et al. Randomized Phase II Trial of Fulvestrant with or without Dasatinib in Postmenopausal Patients with Hormone Receptor-Positive Metastatic Breast Cancer Previously Treated with an Aromatase Inhibitor. Cancer Res 2011;71:PD01-01. [Crossref]
- Mayer EL, Baurain JF, Sparano J, et al. A phase 2 trial of dasatinib in patients with advanced HER2-positive and/or hormone receptor-positive breast cancer. Clin Cancer Res 2011;17:6897-904. [Crossref] [PubMed]
- Campone M, Bondarenko I, Brincat S, et al. Phase II study of single-agent bosutinib, a Src/Abl tyrosine kinase inhibitor, in patients with locally advanced or metastatic breast cancer pretreated with chemotherapy. Ann Oncol 2012;23:610-7. [Crossref] [PubMed]
- Somlo G, Atzori F, Strauss LC, et al. Dasatinib plus capecitabine for advanced breast cancer: safety and efficacy in phase I study CA180004. Clin Cancer Res 2013;19:1884-93. [Crossref] [PubMed]
- Dasatinib-letrozole gets split verdict. Cancer Discov 2014;4:138-9. [Crossref] [PubMed]
- Moy B, Neven P, Lebrun F, et al. Bosutinib in combination with the aromatase inhibitor exemestane: a phase II trial in postmenopausal women with previously treated locally advanced or metastatic hormone receptor-positive/HER2-negative breast cancer. Oncologist 2014;19:346-7. [Crossref] [PubMed]
- Mitri Z, Nanda R, Blackwell K, et al. TBCRC-010: Phase I/II Study of Dasatinib in Combination with Zoledronic Acid for the Treatment of Breast Cancer Bone Metastasis. Clin Cancer Res 2016;22:5706-12. [Crossref] [PubMed]
- Ocana Fernandez A, Ruiz Borrego M, Martin MG, et al. A phase II trial of dasatinib (D) in combination with trastuzumab (T) and paclitaxel (P) in the first line treatment of HER2 positive metastatic breast cancer (MBC) patients (pts): GEICAM/2010-04. Ann Oncol 2017;28:mdx365.002.
- Jin MH, Nam AR, Park JE, et al. Resistance Mechanism against Trastuzumab in HER2-Positive Cancer Cells and Its Negation by Src Inhibition. Mol Cancer Ther 2017;16:1145-54. [Crossref] [PubMed]
- Hoehn D, Cortes JE, Medeiros LJ, et al. Multiparameter Analysis of Off-Target Effects of Dasatinib on Bone Homeostasis in Patients With Newly Diagnosed Chronic Myelogenous Leukemia. Clin Lymphoma Myeloma Leuk 2016;16 Suppl:S86-92. [Crossref] [PubMed]
- Wang J, Pendergast AM. The Emerging Role of ABL Kinases in Solid Tumors. Trends Cancer 2015;1:110-23. [Crossref] [PubMed]
- Zhu Y, Wang Y, Guan B, et al. C-kit and PDGFRA gene mutations in triple negative breast cancer. Int J Clin Exp Pathol 2014;7:4280-5. [PubMed]
- Sgroi V, Bassanelli M, Roberto M, et al. Complete response in advanced breast cancer patient treated with a combination of capecitabine, oral vinorelbine and dasatinib. Exp Hematol Oncol 2018;7:2. [Crossref] [PubMed]


